Expression of GABAergic receptors in mouse taste receptor cells
- PMID: 21049022
- PMCID: PMC2964312
- DOI: 10.1371/journal.pone.0013639
Expression of GABAergic receptors in mouse taste receptor cells
Abstract
Background: Multiple excitatory neurotransmitters have been identified in the mammalian taste transduction, with few studies focused on inhibitory neurotransmitters. Since the synthetic enzyme glutamate decarboxylase (GAD) for gamma-aminobutyric acid (GABA) is expressed in a subset of mouse taste cells, we hypothesized that other components of the GABA signaling pathway are likely expressed in this system. GABA signaling is initiated by the activation of either ionotropic receptors (GABA(A) and GABA(C)) or metabotropic receptors (GABA(B)) while it is terminated by the re-uptake of GABA through transporters (GATs).
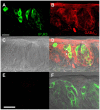
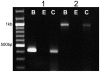
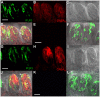
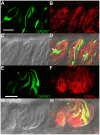
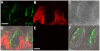
Methodology/principal findings: Using reverse transcriptase-PCR (RT-PCR) analysis, we investigated the expression of different GABA signaling molecules in the mouse taste system. Taste receptor cells (TRCs) in the circumvallate papillae express multiple subunits of the GABA(A) and GABA(B) receptors as well as multiple GATs. Immunocytochemical analyses examined the distribution of the GABA machinery in the circumvallate papillae. Both GABA(A)-and GABA(B)- immunoreactivity were detected in the peripheral taste receptor cells. We also used transgenic mice that express green fluorescent protein (GFP) in either the Type II taste cells, which can respond to bitter, sweet or umami taste stimuli, or in the Type III GAD67 expressing taste cells. Thus, we were able to identify that GABAergic receptors are expressed in some Type II and Type III taste cells. Mouse GAT4 labeling was concentrated in the cells surrounding the taste buds with a few positively labeled TRCs at the margins of the taste buds.
Conclusions/significance: The presence of GABAergic receptors localized on Type II and Type III taste cells suggests that GABA is likely modulating evoked taste responses in the mouse taste bud.
Conflict of interest statement
Figures





References
-
- Delay RJ, Roper SD. Ultrastructure of taste cells and synapses in the mudpuppy Necturus maculosus. J Comp Neurol. 1988;277:268–280. - PubMed
-
- Kinnamon JC, Sherman TA, Roper SD. Ultrastructure of mouse vallate taste buds: III. Patterns of synaptic connectivity. J Comp Neurol. 1988;270:1–10, 56-17. - PubMed
-
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

