Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial
- PMID: 21049059
- PMCID: PMC2964287
- DOI: 10.1371/journal.pntd.0000709
Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial
Abstract
Background: Visceral leishmaniasis (VL) is a major health problem in developing countries. The untreated disease is fatal, available treatment is expensive and often toxic, and drug resistance is increasing. Improved treatment options are needed. Paromomycin was shown to be an efficacious first-line treatment with low toxicity in India.
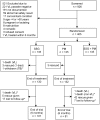
Methods: This was a 3-arm multicentre, open-label, randomized, controlled clinical trial to compare three treatment regimens for VL in East Africa: paromomycin sulphate (PM) at 15 mg/kg/day for 21 days versus sodium stibogluconate (SSG) at 20 mg/kg/day for 30 days; and the combination of both dose regimens for 17 days. The primary efficacy endpoint was cure based on parasite-free tissue aspirates taken 6 months after treatment.
Findings: Overall, 135 patients per arm were enrolled at five centres in Sudan (2 sites), Kenya (1) and Ethiopia (2), when the PM arm had to be discontinued due to poor efficacy. The trial has continued with the higher dose of PM as well as the combination of PM and SSG arms. These results will be reported later. Baseline patient characteristics were similar among treatment arms. The overall cure with PM was significantly inferior to that with SSG (63.8% versus 92.2%; difference 28.5%, 95%CI 18.8% to 38.8%, p<0.001). The efficacy of PM varied among centres and was significantly lower in Sudan (14.3% and 46.7%) than in Kenya (80.0%) and Ethiopia (75.0% and 96.6%). No major safety issues with PM were identified.
Conclusion: The efficacy of PM at 15 mg/kg/day for 21 days was inadequate, particularly in Sudan. The efficacy of higher doses and the combination treatment warrant further studies.
Conflict of interest statement
Manica Balasegaram is employed by DNDi as a Project Manager. Sally Ellis is employed by DNDi as a Clinical Coordinator. Moses Alobo is employed by DNDi as a Clinical Trial Manager. Catherine Royce and John Kinuthia work for DNDi. Marius Mueller and Yousif Koummuki work for MSF.
Figures
References
-
- World Health Organization. Fifth Consultative Meeting on Leishmania/HIV Coinfection, Addis Ababa, Ethiopia. Geneva, Switzerland: World Health Organization; 2007.
-
- Desjeux P. Leishmaniasis. Public health aspects and control. Clin Dermatol. 1996;14:417–423. - PubMed
-
- Rijal S, Chappuis F, Singh R, Boelaert M, Loutan L, et al. Sodium stibogluconate cardiotoxicity and safety of generics. Trans R Soc Trop Med Hyg. 2003;97:597598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

