Paromomycin for the treatment of visceral leishmaniasis in Sudan: a randomized, open-label, dose-finding study
- PMID: 21049063
- PMCID: PMC2964291
- DOI: 10.1371/journal.pntd.0000855
Paromomycin for the treatment of visceral leishmaniasis in Sudan: a randomized, open-label, dose-finding study
Abstract
Background: A recent study has shown that treatment of visceral leishmaniasis (VL) with the standard dose of 15 mg/kg/day of paromomycin sulphate (PM) for 21 days was not efficacious in patients in Sudan. We therefore decided to test the efficacy of paramomycin for a longer treatment duration (15 mg/kg/day for 28 days) and at the higher dose of 20 mg/kg/day for 21 days.
Methods: This randomized, open-label, dose-finding, phase II study assessed the two above high-dose PM treatment regimens. Patients with clinical features and positive bone-marrow aspirates for VL were enrolled. All patients received their assigned courses of PM intramuscularly and adverse events were monitored. Parasite clearance in bone-marrow aspirates was tested by microscopy at end of treatment (EOT, primary efficacy endpoint), 3 months (in patients who were not clinically well) and 6 months after EOT (secondary efficacy endpoint). Pharmacokinetic data were obtained from a subset of patients weighing over 30 kg.
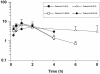
Findings: 42 patients (21 per group) aged between 4 and 60 years were enrolled. At EOT, 85% of patients (95% confidence interval [CI]: 63.7% to 97.0%) in the 20 mg/kg/day group and 90% of patients (95% CI: 69.6% to 98.8%) in the 15 mg/kg/day group had parasite clearance. Six months after treatment, efficacy was 80.0% (95% CI: 56.3% to 94.3%) and 81.0% (95% CI: 58.1% to 94.6%) in the 20 mg/kg/day and 15 mg/kg/day groups, respectively. There were no serious adverse events. Pharmacokinetic profiles suggested a difference between the two doses, although numbers of patients recruited were too few to make it significant (n = 3 and n = 6 in the 20 mg/kg/day and 15 mg/kg/day groups, respectively).
Conclusion: Data suggest that both high dose regimens were more efficacious than the standard 15 mg/kg/day PM for 21 days and could be further evaluated in phase III studies in East Africa.
Trial registration: ClinicalTrials.gov NCT00255567.
Conflict of interest statement
CR was working for DNDi at the time of the study. MB is currently working at DNDi.
Figures
Similar articles
-
Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial.PLoS Negl Trop Dis. 2010 Oct 26;4(10):e709. doi: 10.1371/journal.pntd.0000709. PLoS Negl Trop Dis. 2010. PMID: 21049059 Free PMC article. Clinical Trial.
-
Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial.PLoS Negl Trop Dis. 2012;6(6):e1674. doi: 10.1371/journal.pntd.0001674. Epub 2012 Jun 19. PLoS Negl Trop Dis. 2012. PMID: 22724029 Free PMC article. Clinical Trial.
-
Safety and efficacy of short course combination regimens with AmBisome, miltefosine and paromomycin for the treatment of visceral leishmaniasis (VL) in Bangladesh.PLoS Negl Trop Dis. 2017 May 30;11(5):e0005635. doi: 10.1371/journal.pntd.0005635. eCollection 2017 May. PLoS Negl Trop Dis. 2017. PMID: 28558062 Free PMC article. Clinical Trial.
-
Paromomycin in the treatment of leishmaniasis.Expert Opin Investig Drugs. 2008 May;17(5):787-94. doi: 10.1517/13543784.17.5.787. Expert Opin Investig Drugs. 2008. PMID: 18447603 Review.
-
Paromomycin.Trans R Soc Trop Med Hyg. 2009 Jul;103(7):653-60. doi: 10.1016/j.trstmh.2008.09.008. Epub 2008 Oct 23. Trans R Soc Trop Med Hyg. 2009. PMID: 18947845 Review.
Cited by
-
Interest in paromomycin for the treatment of visceral leishmaniasis (kala-azar).Ther Clin Risk Manag. 2012;8:323-8. doi: 10.2147/TCRM.S30139. Epub 2012 Jun 22. Ther Clin Risk Manag. 2012. PMID: 22802694 Free PMC article.
-
Immunogenicity and immune modulatory effects of in silico predicted L. donovani candidate peptide vaccines.Hum Vaccin Immunother. 2012 Dec 1;8(12):1769-74. doi: 10.4161/hv.21881. Epub 2012 Aug 24. Hum Vaccin Immunother. 2012. PMID: 22922767 Free PMC article.
-
Visceral leishmaniasis-hepatitis B/C coinfections: a rising necessity to triage patients for treatment.Ann Saudi Med. 2014 Mar-Apr;34(2):143-6. doi: 10.5144/0256-4947.2014.143. Ann Saudi Med. 2014. PMID: 24894783 Free PMC article.
-
Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis.Chem Rev. 2014 Nov 26;114(22):11305-47. doi: 10.1021/cr500365f. Epub 2014 Nov 3. Chem Rev. 2014. PMID: 25365529 Free PMC article. Review. No abstract available.
-
Molecular Basis of the Leishmanicidal Activity of the Antidepressant Sertraline as a Drug Repurposing Candidate.Antimicrob Agents Chemother. 2018 Nov 26;62(12):e01928-18. doi: 10.1128/AAC.01928-18. Print 2018 Dec. Antimicrob Agents Chemother. 2018. PMID: 30297370 Free PMC article.
References
-
- Desjeux P. Leishmaniasis. Public health aspects and control. Clin Dermatol. 1996;14:417–423. - PubMed
-
- World Health Organization. Leishmaniasis. 2009. http://www.who.int/leishmaniasis/disease_epidemiology/en/index.html. Accessed 2009 Nov 26.
-
- Rijal S, Chappuis F, Singh R, Boelaert M, Loutan L, et al. Sodium stibogluconate cardiotoxicity and safety of generics. Trans R Soc Trop Med Hyg. 2003;97:597–598. - PubMed
-
- Thakur CP, Sinha GP, Pandey AK, Kumar N, Kumar P, et al. Do the diminishing efficacy and increasing toxicity of sodium stibogluconate in the treatment of visceral leishmaniasis in Bihar, India, justify its continued use as a first-line drug? An observational study of 80 cases. Ann Trop Med Parasitol. 1998;92:561–569. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

