MALDI-TOF MS in Prenatal Genomics
- PMID: 21049077
- PMCID: PMC2941831
- DOI: 10.1159/000223098
MALDI-TOF MS in Prenatal Genomics
Abstract
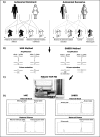
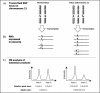
Prenatal diagnosis aims either to provide the reassurance to the couples at risk of having an affected child by timely appropriate therapy or to give the parents a chance to decide the fate of the unborn babies with health problems. Invasive prenatal diagnosis (IPD) is accurate, however, carrying a risk of miscarriage. Non-invasive prenatal diagnosis (NIPD) has been developed based on the existing of fetal genetic materials in maternal circulation; however, a minority fetal DNA in majority maternal background DNA hinders the detections of fetal traits. Different protocols and assays, such as homogenous MassEXTEND (hME), single allele base extension reaction (SABER), precise measuring copy number variation of each allele, and quantitative methylation and expression analysis using the high-throughput sensitive matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS), allow NIPD for single gene disorders, fetal blood group genotyping and fetal aneuploidies as well as the development of fetal gender-independent biomarkers in maternal circulation for management of pathological pregnancies. In this review, we summarise the use of MALDI-TOF MS in prenatal genomics.
Die pränatale Diagnostik zielt darauf ab, entweder Paaren, bei denen das Risiko eines betroffenen Kindes besteht, die Rückversicherung für eine rechtzeitige angemessene Therapie zu geben, oder Eltern die Chance zu geben über das Schicksal von ungeborenen Kindern mit schwerwiegenden gesundheitlichen Einschränkungen zu entscheiden. Die invasive pränatale Diagnostik (IPD) ist präzise, birgt aber das Risiko einer Fehlgeburt. Die non-invasive pränatale Diagnostik (NIPD) wurde auf der Grundlage des vorhandenen fetalen genetischen Materials im mütterlichen Blutkreislauf entwickelt. Allerdings erschwert die geringe Menge fetaler DNA innerhalb der großen Menge an mütterlicher DNA den Nachweis von fetalen Charakteristika. Verschiedene Protokolle und Assays – z.B. «homogenous MassEXTEND» (hME), ‘single allele base extension reaction’ (SABER), präzise Messung der Variation der Kopienzahl eines jeden Allels und quantitiative Methylierung und Expressionsanalyse mithilfe der ‘matrix-assisted laser desorption/ionization-time of flight mass spectrometry’ (MALDI-TOF MS) – ermöglichen eine NIPD von einzelnen Genstörungen, fetaler Blutgruppengenotypisierung und fetalen Aneu-ploidien ebenso wie die Entwicklung von fetalen, geschlechtsunabhängigen Biomarkern in der mütterlichen Zirkulation für das Management pathologischer Schwangerschaften. Die vorliegende Arbeit gibt einen Überblick zur Verwendung von MALDI-TOF MS in der pränatalen Diagnostik.
Figures
References
-
- Buckley F, Buckley SJ. Costs of prenatal genetic screening. Lancet. 2008;372:1805. - PubMed
-
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. 2008;57:1–32. - PubMed
-
- Kuppermann M, Norton ME. Prenatal testing guidelines: time for a new approach. Gynecol Obstet Invest. 2005;60:6–10. - PubMed
-
- Schmid M, Drahonsky R, Fast-Hirsch C, Baumuhlner K, Husslein P, Blaicher W. Timing of referral for prenatal genetic counselling. Prenat Diagn. 2009;29:156–159. - PubMed
-
- Anderson CL, Brown CE. Fetal chromosomal abnormalities: antenatal screening and diagnosis. Am Fam Physician. 2009;79:117–123. - PubMed
LinkOut - more resources
Full Text Sources

