Theory of coupled electron and proton transfer reactions
- PMID: 21049940
- PMCID: PMC3005854
- DOI: 10.1021/cr1001436
Theory of coupled electron and proton transfer reactions
Figures
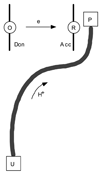
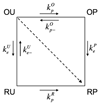







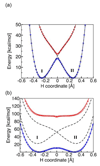
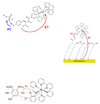

References
-
- Nicholls DG, Ferguson S. Bioenergetics 2. San Diego: Academic Press; 1992.
-
- Cramer WA, Knaff DB. Energy transduction in biological membranes. New York: Springer-Verlag; 1990.
-
- Skulachev VP. Membrane bioenergetics. New York: Springer-Verlag; 1988.
-
- Wikström M. Curr. Opin. Struct. Biol. 1998;8:480. - PubMed
-
- Michel H. Biochemistry. 1999;38:15129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

