Cell-based screening using high-throughput flow cytometry
- PMID: 21050072
- PMCID: PMC3045571
- DOI: 10.1089/adt.2010.0308
Cell-based screening using high-throughput flow cytometry
Abstract
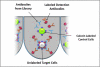
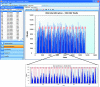
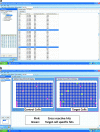
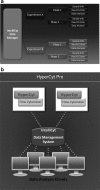
This review describes the use of high-throughput flow cytometry for performing multiplexed cell-based and bead-based screens. With the many advances in cell-based analysis and screening, flow cytometry has historically been underutilized as a screening tool largely due to the limitations in handling large numbers of samples. However, there has been a resurgence in the use of flow cytometry due to a combination of innovations around instrumentation and a growing need for cell-based and bead-based applications. The HTFC™ Screening System (IntelliCyt Corporation, Albuquerque, NM) is a novel flow cytometry-based screening platform that incorporates a fast sample-loading technology, HyperCyt®, with a two-laser, six-parameter flow cytometer and powerful data analysis capabilities. The system is capable of running multiplexed screening assays at speeds of up to 40 wells per minute, enabling the processing of a 96- and 384-well plates in as little as 3 and 12 min, respectively. Embedded in the system is HyperView®, a data analysis software package that allows rapid identification of hits from multiplexed high-throughput flow cytometry screening campaigns. In addition, the software is incorporated into a server-based data management platform that enables seamless data accessibility and collaboration across multiple sites. High-throughput flow cytometry using the HyperCyt technology has been applied to numerous assay areas and screening campaigns, including efflux transporters, whole cell and receptor binding assays, functional G-protein-coupled receptor screening, in vitro toxicology, and antibody screening.
Figures



Similar articles
-
Advances in laser scanning imaging cytometry for high-content screening.Assay Drug Dev Technol. 2015 Mar;13(2):66-78. doi: 10.1089/adt.2014.607. Epub 2015 Feb 5. Assay Drug Dev Technol. 2015. PMID: 25654565 Review.
-
Microfluidic cell culture systems for drug research.Lab Chip. 2010 Apr 21;10(8):939-56. doi: 10.1039/b921695b. Epub 2010 Jan 21. Lab Chip. 2010. PMID: 20358102 Review.
-
A serial dilution microfluidic device for cytotoxicity assays.Conf Proc IEEE Eng Med Biol Soc. 2006;2006:2836-9. doi: 10.1109/IEMBS.2006.259270. Conf Proc IEEE Eng Med Biol Soc. 2006. PMID: 17946141
-
Microfluidic devices for cell based high throughput screening.Lab Chip. 2010 Feb 7;10(3):341-8. doi: 10.1039/b918291h. Epub 2009 Nov 27. Lab Chip. 2010. PMID: 20091006
-
Webcam-based flow cytometer using wide-field imaging for low cell number detection at high throughput.Analyst. 2014 Sep 7;139(17):4322-9. doi: 10.1039/c4an00669k. Analyst. 2014. PMID: 24995370
Cited by
-
Nanoparticle labeling of bone marrow-derived rat mesenchymal stem cells: their use in differentiation and tracking.Biomed Res Int. 2015;2015:298430. doi: 10.1155/2015/298430. Epub 2015 Jan 14. Biomed Res Int. 2015. PMID: 25654092 Free PMC article.
-
The promise of single-cell mechanophenotyping for clinical applications.Biomicrofluidics. 2020 Jun 9;14(3):031301. doi: 10.1063/5.0010800. eCollection 2020 May. Biomicrofluidics. 2020. PMID: 32566069 Free PMC article.
-
Very rapid cloning, expression and identifying specificity of T-cell receptors for T-cell engineering.PLoS One. 2020 Feb 10;15(2):e0228112. doi: 10.1371/journal.pone.0228112. eCollection 2020. PLoS One. 2020. PMID: 32040512 Free PMC article.
-
High-throughput screen for the chemical inhibitors of antiapoptotic bcl-2 family proteins by multiplex flow cytometry.Assay Drug Dev Technol. 2011 Oct;9(5):465-74. doi: 10.1089/adt.2010.0363. Epub 2011 May 11. Assay Drug Dev Technol. 2011. PMID: 21561376 Free PMC article.
-
The role of alemtuzumab in the development of secondary autoimmunity in multiple Sclerosis: a systematic review.J Neuroinflammation. 2024 Nov 1;21(1):281. doi: 10.1186/s12974-024-03263-9. J Neuroinflammation. 2024. PMID: 39487492 Free PMC article.
References
-
- Feng Y. Mitchison TJ. Bender A. Young DW. Tallarico JA. Multi-parameter phenotypic profiling: using cellular effects to characterize small-molecule compounds. Nat Rev Drug Discov. 2009;8:567–578. - PubMed
-
- Edwards BS. Kuckuck FW. Prossnitz ER. Ransom JT. Sklar LA. HTPS flow cytometry: a novel platform for automated high throughput drug discovery and characterization. J Biomol Screen. 2001;6:83–90. - PubMed
-
- Edwards BS. Young SM. Saunders MJ. Bologa C. Oprea TI. Ye RD. Prossnitz ER. Graves SW. Sklar LA. High-throughput flow cytometry for drug discovery. Expert Opin Drug Discov. 2007;2:685–696. - PubMed
-
- Roman DL. Talbot JN. Roof RA. Sunahara RK. Traynor JR. Neubig RR. Identification of small-molecule inhibitors of RGS4 using a high-throughput flow cytometry protein interaction assay. Mol Pharmacol. 2007;71:169–175. - PubMed
-
- Simons PC. Shi M. Foutz T. Cimino DF. Lewis J. Buranda T. Lim W. Neubig RR. McIntire WE. Garrison J. Prossnitz E. Sklar LA. Ligand–receptor–G-protein molecular assemblies on beads for mechanistic studies and screening by flow cytometry. Mol Pharmacol. 2003;64:1227–1238. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

