Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections
- PMID: 21050275
- PMCID: PMC2970673
- DOI: 10.1111/j.1460-9568.2010.07337.x
Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections
Abstract
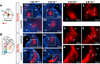
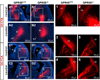
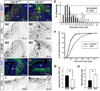
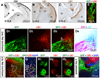
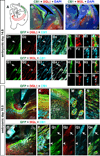
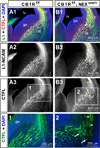
A role for endocannabinoid signaling in neuronal morphogenesis as the brain develops has recently been suggested. Here we used the developing somatosensory circuit as a model system to examine the role of endocannabinoid signaling in neural circuit formation. We first show that a deficiency in cannabinoid receptor type 1 (CB(1)R), but not G-protein-coupled receptor 55 (GPR55), leads to aberrant fasciculation and pathfinding in both corticothalamic and thalamocortical axons despite normal target recognition. Next, we localized CB(1)R expression to developing corticothalamic projections and found little if any expression in thalamocortical axons, using a newly established reporter mouse expressing GFP in thalamocortical projections. A similar thalamocortical projection phenotype was observed following removal of CB(1)R from cortical principal neurons, clearly demonstrating that CB(1)R in corticothalamic axons was required to instruct their complimentary connections, thalamocortical axons. When reciprocal thalamic and cortical connections meet, CB(1)R-containing corticothalamic axons are intimately associated with elongating thalamocortical projections containing DGLβ, a 2-arachidonoyl glycerol (2-AG) synthesizing enzyme. Thus, 2-AG produced in thalamocortical axons and acting at CB(1)Rs on corticothalamic axons is likely to modulate axonal patterning. The presence of monoglyceride lipase, a 2-AG degrading enzyme, in both thalamocortical and corticothalamic tracts probably serves to restrict 2-AG availability. In summary, our study provides strong evidence that endocannabinoids are a modulator for the proposed 'handshake' interactions between corticothalamic and thalamocortical axons, especially for fasciculation. These findings are important in understanding the long-term consequences of alterations in CB(1)R activity during development, a potential etiology for the mental health disorders linked to prenatal cannabis use.
© 2010 The Authors. European Journal of Neuroscience © 2010 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures

Similar articles
-
Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: evidence that cortical and thalamic axons interact and guide each other.J Comp Neurol. 2002 May 20;447(1):8-17. doi: 10.1002/cne.10219. J Comp Neurol. 2002. PMID: 11967891
-
A forward genetic screen with a thalamocortical axon reporter mouse yields novel neurodevelopment mutants and a distinct emx2 mutant phenotype.Neural Dev. 2011 Jan 7;6:3. doi: 10.1186/1749-8104-6-3. Neural Dev. 2011. PMID: 21214893 Free PMC article.
-
Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding.J Neurosci. 2010 Oct 20;30(42):13992-4007. doi: 10.1523/JNEUROSCI.2126-10.2010. J Neurosci. 2010. PMID: 20962221 Free PMC article.
-
Conserved developmental algorithms during thalamocortical circuit formation in mammals and reptiles.Novartis Found Symp. 2000;228:148-66; discussion 166-72. doi: 10.1002/0470846631.ch11. Novartis Found Symp. 2000. PMID: 10929321 Review.
-
The endocannabinoid-CB receptor system: Importance for development and in pediatric disease.Neuro Endocrinol Lett. 2004 Feb-Apr;25(1-2):24-30. Neuro Endocrinol Lett. 2004. PMID: 15159678 Review.
Cited by
-
Marijuana, Spice 'herbal high', and early neural development: implications for rescheduling and legalization.Drug Test Anal. 2013 Jan;5(1):27-45. doi: 10.1002/dta.1390. Epub 2012 Aug 13. Drug Test Anal. 2013. PMID: 22887867 Free PMC article. Review.
-
Diacylglycerol lipase α manipulation reveals developmental roles for intercellular endocannabinoid signaling.Sci Rep. 2013;3:2093. doi: 10.1038/srep02093. Sci Rep. 2013. PMID: 23806960 Free PMC article.
-
Endocannabinoid signaling in the central nervous system.Glia. 2023 Jan;71(1):5-35. doi: 10.1002/glia.24280. Epub 2022 Oct 29. Glia. 2023. PMID: 36308424 Free PMC article.
-
Cannabinoid-induced actomyosin contractility shapes neuronal morphology and growth.Elife. 2014 Sep 15;3:e03159. doi: 10.7554/eLife.03159. Elife. 2014. PMID: 25225054 Free PMC article.
-
The CB(1) cannabinoid receptor drives corticospinal motor neuron differentiation through the Ctip2/Satb2 transcriptional regulation axis.J Neurosci. 2012 Nov 21;32(47):16651-65. doi: 10.1523/JNEUROSCI.0681-12.2012. J Neurosci. 2012. PMID: 23175820 Free PMC article.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Curr Opin Neurobiol. 2003;13:440–445. - PubMed
-
- Antonelli T, Tanganelli S, Tomasini MC, Finetti S, Trabace L, Steardo L, Sabino V, Carratu MR, Cuomo V, Ferraro L. Long-term effects on cortical glutamate release induced by prenatal exposure to the cannabinoid receptor agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinyl-methyl)pyrrolo[1,2,3-de]-1, 4-benzoxazin-6-yl]-1-naphthalenylmethanone: an in vivo microdialysis study in the awake rat. Neuroscience. 2004;124:367–375. - PubMed
-
- Antonelli T, Tomasini MC, Tattoli M, Cassano T, Tanganelli S, Finetti S, Mazzoni E, Trabace L, Steardo L, Cuomo V, Ferraro L. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb Cortex. 2005;15:2013–2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

