Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis
- PMID: 21050433
- PMCID: PMC3046511
- DOI: 10.1186/ar3180
Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis
Abstract
Introduction: Microvasculopathy is one of the characteristic features in patients with systemic sclerosis (SSc), but underlying mechanisms still remain uncertain. In this study, we evaluated the potential involvement of monocytic endothelial progenitor cells (EPCs) in pathogenic processes of SSc vasculopathy, by determining their number and contribution to blood vessel formation through angiogenesis and vasculogenesis.
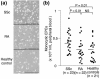
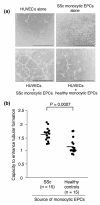
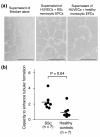
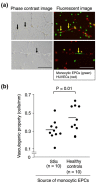
Methods: Monocytic EPCs were enriched and enumerated using a culture of peripheral blood mononuclear cells and platelets on fibronectin in 23 patients with SSc, 22 patients with rheumatoid arthritis (RA), and 21 healthy controls. To assess the capacity of monocytic EPCs to promote vascular formation and the contribution of vasculogenesis to this process, we used an in vitro co-culture system with human umbilical vein endothelial cells (HUVECs) on Matrigel® and an in vivo murine tumor neovascularization model.
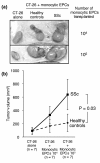
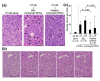
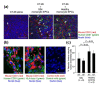
Results: Monocytic EPCs were significantly increased in SSc patients than in RA patients or healthy controls (P = 0.01 for both comparisons). Monocytic EPCs derived from SSc patients promoted tubular formation in Matrigel® cultures more than those from healthy controls (P = 0.007). Transplantation of monocytic EPCs into immunodeficient mice resulted in promotion of tumor growth and blood vessel formation, and these properties were more prominent in SSc than healthy monocytic EPCs (P = 0.03 for both comparisons). In contrast, incorporation of SSc monocytic EPCs into the tubular structure was less efficient in vitro and in vivo, compared with healthy monocytic EPCs.
Conclusions: SSc patients have high numbers of aberrant circulating monocytic EPCs that exert enhanced angiogenesis but are impaired in vasculogenesis. However, these cells apparently cannot overcome the anti-angiogenic environment that characterizes SSc-affected tissues.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

