Antiproliferative and pro-apoptotic effects afforded by novel Src-kinase inhibitors in human neuroblastoma cells
- PMID: 21050441
- PMCID: PMC2992519
- DOI: 10.1186/1471-2407-10-602
Antiproliferative and pro-apoptotic effects afforded by novel Src-kinase inhibitors in human neuroblastoma cells
Abstract
Background: Neuroblastoma (NB) is the second most common solid malignancy of childhood that usually undergoes rapid progression with a poor prognosis upon metastasis. The Src-family tyrosine kinases (SFKs) are a group of proteins involved in cancer development and invasiveness that seem to play an important role in the NB carcinogenesis.
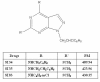
Methods: To determine cell proliferation, the growth rate was evaluated by both MTT test and cells counted. Analysis of DNA content was performed for the evaluation of the cell cycle and apoptosis. To characterize the mechanisms underlying the antiproliferative effects induced by SI 34, a novel pyrazolo-pyrimidine derivative provided with Src inhibitory activity, the involvement of some cellular pathways that are important for cell proliferation and survival was investigated by western blot assays. In particular, the contribution of cyclins, Src and ERK were examined. Finally, experiments of cell adhesion and invasiveness were performed.
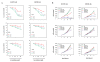
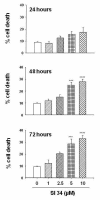
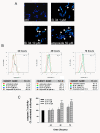
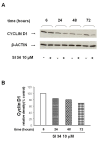
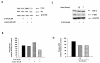
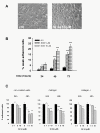
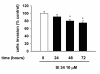
Results: Treatment of SH-SY5Y human NB cells and CHP100 human neuroepithelioma (NE) cultures with three novel pyrazolo[3,4-d]pyrimidine derivatives, namely SI 34, SI 35 and SI 83, inhibits the cell proliferation in a time and concentration-dependent manner. The maximal effect was obtained after 72 hours incubation with SI 34 10 μM. Fluorescence microscopy experiments, flow cytometry analysis and determination of caspase-3 activity by fluorimetric assays showed that SI 34 induced SH-SY5Y apoptosis. Moreover, SI 34 determined cell cycle arrest at the G0/G1 phase, paralleled by a decreased expression of cyclin D1. Furthermore, our data indicate that SI 34 reduces the SH-SY5Y cells adhesion and invasiveness. Evidence that SI 34 inhibits the Src and the ERK-phosphorylation, suggests the mechanism through which it exerts its effects in SH-SY5Y cells.
Conclusions: Our study shows the ability of this pyrazolo-pyrimidine Src inhibitor in reducing the growth and the invasiveness of human NB cells, suggesting a promising role as novel drug in the treatment of neuroblastoma.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

