Innate signals in mucosal immunoglobulin class switching
- PMID: 21050939
- PMCID: PMC3047474
- DOI: 10.1016/j.jaci.2010.09.026
Innate signals in mucosal immunoglobulin class switching
Abstract
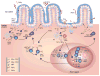
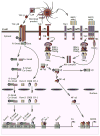
The intestinal mucosa contains large communities of commensal bacteria that process otherwise indigestible food components, synthesize essential vitamins, stimulate the maturation of the immune system, and form an ecologic niche that prevents the growth of pathogenic species. Conversely, the intestine provides the commensals with a stable habitat rich in energy derived from the ingested food. A delicate homeostatic balance maintains this mutualistic relationship without triggering a destructive inflammatory response. Commensals orchestrate intestinal homeostasis by entertaining an intimate dialogue with epithelial cells and immune cells lodged in the mucosa. Such a dialogue generates finely tuned signaling programs that ensure a state of hyporesponsiveness against noninvasive commensals and a state of active readiness against invasive pathogens. In this dialogue epithelial cells function as "interpreters" that continuously translate microbial messages to "instruct" immune cells as to the antigenic composition of the intestinal lumen. This education process initiates sophisticated defensive strategies that comprise massive production of IgA, a noninflammatory mucosal antibody class that generates immunity while preserving homeostasis.
Copyright © 2010 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures
References
-
- Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. - PubMed
-
- Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, et al. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. - PubMed
-
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–71. - PubMed
-
- Muramatsu M, Nagaoka H, Shinkura R, Begum NA, Honjo T. Discovery of activation-induced cytidine deaminase, the engraver of antibody memory. Adv Immunol. 2007;94:1–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

