Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal
- PMID: 21051498
- PMCID: PMC3033719
- DOI: 10.1124/jpet.110.173823
Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal
Abstract
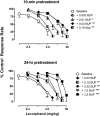
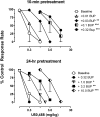
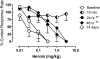
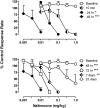
The dual antagonist effects of the mixed-action μ-opioid partial agonist/κ-opioid antagonist buprenorphine have not been previously compared in behavioral studies, and it is unknown whether they are comparably modified by chronic exposure. To address this question, the dose-related effects of levorphanol, trans-(-)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl] benzeneacetamide (U50,488), heroin, and naltrexone on food-maintained behavior in rhesus monkeys were studied after acute and chronic treatment with buprenorphine (0.3 mg/kg/day). In acute studies, the effects of levorphanol and U50,488 were determined at differing times after buprenorphine (0.003-10.0 mg/kg i.m.). Results show that buprenorphine produced similar, dose-dependent rightward shifts of the levorphanol and U50,488 dose-response curves that persisted for ≥ 24 h after doses larger than 0.1 mg/kg buprenorphine. During chronic treatment with buprenorphine, the effects of levorphanol, U50,488, heroin, and naltrexone were similarly determined at differing times (10 min to 48 h) after intramuscular injection. Overall, results show that buprenorphine produced comparable 3- to 10-fold rightward shifts in the U50,488 dose-response curve under both acute and chronic conditions, but that chronic buprenorphine produced larger (10- to ≥ 30-fold) rightward shifts in the heroin dose-effect function than observed acutely. Naltrexone decreased operant responding in buprenorphine-treated monkeys, and the position of the naltrexone dose-effect curve shifted increasingly to the left as the time after daily buprenorphine treatment increased from 10 min to 48 h. These results suggest that the μ-antagonist, but not the κ-antagonist, effects of buprenorphine are augmented during chronic treatment. In addition, the leftward shift of the naltrexone dose-effect function suggests that daily administration of 0.3 mg/kg buprenorphine is adequate to produce opioid dependence.
Figures
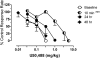
Similar articles
-
Thienorphine: receptor binding and behavioral effects in rhesus monkeys.J Pharmacol Exp Ther. 2007 Apr;321(1):227-36. doi: 10.1124/jpet.106.113290. Epub 2007 Jan 12. J Pharmacol Exp Ther. 2007. PMID: 17220427
-
Kappa antagonist properties of buprenorphine in the shock titration procedure.Eur J Pharmacol. 1988 Oct 26;156(1):77-86. doi: 10.1016/0014-2999(88)90149-5. Eur J Pharmacol. 1988. PMID: 2850212
-
Kappa opioid antagonist effects of the novel kappa antagonist 5'-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys.Psychopharmacology (Berl). 2002 Oct;163(3-4):412-9. doi: 10.1007/s00213-002-1038-x. Epub 2002 Mar 13. Psychopharmacology (Berl). 2002. PMID: 12373442
-
Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys.J Pharmacol Exp Ther. 1999 Dec;291(3):1113-20. J Pharmacol Exp Ther. 1999. PMID: 10565831 Free PMC article.
-
Levorphanol.2019 Apr 25. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2019 Apr 25. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31643300 Free Books & Documents. Review.
Cited by
-
Buprenorphine prevents stress-induced blunting of nucleus accumbens dopamine response and approach behavior to food reward in mice.Neurobiol Stress. 2019 Jun 12;11:100182. doi: 10.1016/j.ynstr.2019.100182. eCollection 2019 Nov. Neurobiol Stress. 2019. PMID: 31304200 Free PMC article.
-
Sex differences in the effectiveness of buprenorphine to decrease rates of responding in rhesus monkeys.Behav Pharmacol. 2019 Jun;30(4):358-362. doi: 10.1097/FBP.0000000000000437. Behav Pharmacol. 2019. PMID: 30212383 Free PMC article.
-
Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence.Psychopharmacology (Berl). 2012 Apr;220(3):559-64. doi: 10.1007/s00213-011-2503-1. Epub 2011 Sep 30. Psychopharmacology (Berl). 2012. PMID: 21960180 Free PMC article. Clinical Trial.
-
Concurrent Assessment of the Antinociceptive and Behaviorally Disruptive Effects of Opioids in Squirrel Monkeys.J Pain. 2018 Jul;19(7):728-740. doi: 10.1016/j.jpain.2018.02.003. Epub 2018 Mar 2. J Pain. 2018. PMID: 29477761 Free PMC article.
-
EPD1504: a novel μ-opioid receptor partial agonist attenuates obsessive-compulsive disorder (OCD)-like behaviors.Front Psychiatry. 2023 Jun 30;14:1170541. doi: 10.3389/fpsyt.2023.1170541. eCollection 2023. Front Psychiatry. 2023. PMID: 37457777 Free PMC article.
References
-
- Adams JU, Holtzman SG. (1990) Tolerance and dependence after continuous morphine infusion from osmotic pumps measured by operant responding in rats. Psychopharmacology 100:451–458 - PubMed
-
- Adams JU, Paronis CA, Holtzman SG. (1990) Assessment of relative intrinsic activity of μ-opioid analgesics in vivo by using β-funaltrexamine. J Pharmacol Exp Ther 255:1027–1032 - PubMed
-
- Berthold CW, 3rd, Moerschbaecher JM. (1988) Tolerance to the effects of buprenorphine on schedule-controlled behavior and analgesia in rats. Pharmacol Biochem Behav 29:393–396 - PubMed
-
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. (1988) Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther 247:47–53 - PubMed
-
- Carey GJ, Bergman J. (2001) Enadoline discrimination in squirrel monkeys: effects of opioid agonists and antagonists. J Pharmacol Exp Ther 297:215–223 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

