In vivo analysis of mouse gastrin gene regulation in enhanced GFP-BAC transgenic mice
- PMID: 21051525
- PMCID: PMC3043646
- DOI: 10.1152/ajpgi.00134.2010
In vivo analysis of mouse gastrin gene regulation in enhanced GFP-BAC transgenic mice
Abstract
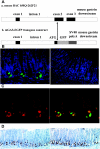
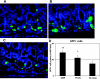
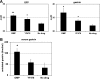
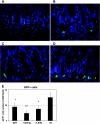
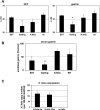
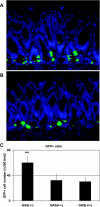
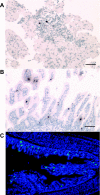
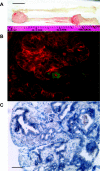
Gastrin is secreted from a subset of neuroendocrine cells residing in the gastric antrum known as G cells, but low levels are also expressed in fetal pancreas and intestine and in many solid malignancies. Although past studies have suggested that antral gastrin is transcriptionally regulated by inflammation, gastric pH, somatostatin, and neoplastic transformation, the transcriptional regulation of gastrin has not previously been demonstrated in vivo. Here, we describe the creation of an enhanced green fluorescent protein reporter (mGAS-EGFP) mouse using a bacterial artificial chromosome that contains the entire mouse gastrin gene. Three founder lines expressed GFP signals in the gastric antrum and the transitional zone to the corpus. In addition, GFP(+) cells could be detected in the fetal pancreatic islets and small intestinal villi, but not in these organs of the adult mice. The administration of acid-suppressive reagents such as proton pump inhibitor omeprazole and gastrin/CCK-2 receptor antagonist YF476 significantly increased GFP signal intensity and GFP(+) cell numbers in the antrum, whereas these parameters were decreased by overnight fasting, octreotide (long-lasting somatostatin ortholog) infusion, and Helicobacter felis infection. GFP(+) cells were also detected in the anterior lobe of the pituitary gland and importantly in the colonic tumor cells induced by administration with azoxymethane and dextran sulfate sodium salt. This transgenic mouse provides a useful tool to study the regulation of mouse gastrin gene in vivo, thus contributing to our understanding of the mechanisms involved in transcriptional control of the gastrin gene.
Figures

References
-
- Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta 1704: 1– 10, 2004 - PubMed
-
- Brand SJ, Fuller PJ. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem 263: 5341– 5347, 1988 - PubMed
-
- Caplin M, Khan K, Savage K, Rode J, Varro A, Michaeli D, Grimes S, Brett B, Pounder R, Dhillon A. Expression and processing of gastrin in hepatocellular carcinoma, fibrolamellar carcinoma and cholangiocarcinoma. J Hepatol 30: 519– 526, 1999 - PubMed
-
- Caplin M, Savage K, Khan K, Brett B, Rode J, Varro A, Dhillon A. Expression and processing of gastrin in pancreatic adenocarcinoma. Br J Surg 87: 1035– 1040, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

