ZFP260 is an inducer of cardiac hypertrophy and a nuclear mediator of endothelin-1 signaling
- PMID: 21051538
- PMCID: PMC3020759
- DOI: 10.1074/jbc.M110.162966
ZFP260 is an inducer of cardiac hypertrophy and a nuclear mediator of endothelin-1 signaling
Abstract
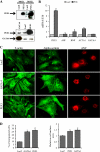
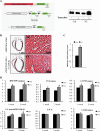
Pressure and volume overload induce hypertrophic growth of postnatal cardiomyocytes and genetic reprogramming characterized by reactivation of a subset of fetal genes. Despite intense efforts, the nuclear effectors of cardiomyocyte hypertrophy remain incompletely defined. Endothelin-1 (ET-1) plays an important role in cardiomyocyte growth and is involved in mediating the neurohormonal effects of mechanical stress. Here, we show that the phenylephrine-induced complex-1 (PEX1), also known as zinc finger transcription factor ZFP260, is essential for cardiomyocyte response to ET-1 as evidenced in cardiomyocytes with PEX1 knockdown. We found that ET-1 enhances PEX1 transcriptional activity via a PKC-dependent pathway which phosphorylates the protein and further potentiates its synergy with GATA4. Consistent with a role for PEX1 in cardiomyocyte hypertrophy, overexpression of PEX1 is sufficient to induce cardiomyocyte hypertrophy in vitro and in vivo. Importantly, transgenic mice with inducible PEX1 expression in the adult heart develop cardiac hypertrophy with preserved heart function. Together, the results identify a novel nuclear effector of ET-1 signaling and suggest that PEX1 may be a regulator of the early stages of cardiac hypertrophy.
Figures



Similar articles
-
COX-2 is involved in ET-1-induced hypertrophy of neonatal rat cardiomyocytes: role of NFATc3.Mol Cell Endocrinol. 2014 Feb 15;382(2):998-1006. doi: 10.1016/j.mce.2013.11.012. Epub 2013 Nov 26. Mol Cell Endocrinol. 2014. PMID: 24291639
-
O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy.Am J Physiol Heart Circ Physiol. 2012 May 15;302(10):H2122-30. doi: 10.1152/ajpheart.00775.2011. Epub 2012 Mar 9. Am J Physiol Heart Circ Physiol. 2012. PMID: 22408028 Free PMC article.
-
Targeted inhibition of ANKRD1 disrupts sarcomeric ERK-GATA4 signal transduction and abrogates phenylephrine-induced cardiomyocyte hypertrophy.Cardiovasc Res. 2015 May 1;106(2):261-71. doi: 10.1093/cvr/cvv108. Epub 2015 Mar 13. Cardiovasc Res. 2015. PMID: 25770146 Free PMC article.
-
Regulation of Cardiac Transcription Factor GATA4 by Post-Translational Modification in Cardiomyocyte Hypertrophy and Heart Failure.Int Heart J. 2016 Dec 2;57(6):672-675. doi: 10.1536/ihj.16-404. Epub 2016 Nov 4. Int Heart J. 2016. PMID: 27818483 Review.
-
GATA transcription factors in the developing and adult heart.Cardiovasc Res. 2004 Aug 1;63(2):196-207. doi: 10.1016/j.cardiores.2004.03.025. Cardiovasc Res. 2004. PMID: 15249177 Review.
Cited by
-
Minority stress and leukocyte gene expression in sexual minority men living with treated HIV infection.Brain Behav Immun. 2018 May;70:335-345. doi: 10.1016/j.bbi.2018.03.016. Epub 2018 Mar 13. Brain Behav Immun. 2018. PMID: 29548994 Free PMC article.
-
Cyclin D2 is a GATA4 cofactor in cardiogenesis.Proc Natl Acad Sci U S A. 2014 Jan 28;111(4):1415-20. doi: 10.1073/pnas.1312993111. Epub 2014 Jan 13. Proc Natl Acad Sci U S A. 2014. PMID: 24474767 Free PMC article.
-
Phosphorylation of GATA4 at serine 105 is required for left ventricular remodelling process in angiotensin II-induced hypertension in rats.Basic Clin Pharmacol Toxicol. 2020 Sep;127(3):178-195. doi: 10.1111/bcpt.13398. Epub 2020 Mar 9. Basic Clin Pharmacol Toxicol. 2020. PMID: 32060996 Free PMC article.
-
Role of endothelin in uteroplacental circulation and fetal vascular function.Curr Vasc Pharmacol. 2013 Sep;11(5):594-605. doi: 10.2174/1570161111311050004. Curr Vasc Pharmacol. 2013. PMID: 24063378 Free PMC article. Review.
References
-
- MacLellan W. R., Schneider M. D. (2000) Annu. Rev. Physiol. 62, 289–319 - PubMed
-
- Olson E. N., Schneider M. D. (2003) Genes Dev. 17, 1937–1956 - PubMed
-
- Frey N., Olson E. N. (2003) Annu. Rev. Physiol. 65, 45–79 - PubMed
-
- Iwanaga Y., Kihara Y., Hasegawa K., Inagaki K., Yoneda T., Kaburagi S., Araki M., Sasayama S. (1998) Circulation 98, 2065–2073 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

