Identification and characterization of a novel-type ferric siderophore reductase from a gram-positive extremophile
- PMID: 21051545
- PMCID: PMC3023520
- DOI: 10.1074/jbc.M110.192468
Identification and characterization of a novel-type ferric siderophore reductase from a gram-positive extremophile
Abstract
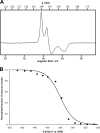
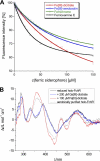
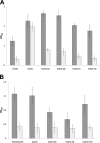
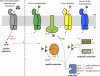
Iron limitation is one major constraint of microbial life, and a plethora of microbes use siderophores for high affinity iron acquisition. Because specific enzymes for reductive iron release in gram-positives are not known, we searched Firmicute genomes and found a novel association pattern of putative ferric siderophore reductases and uptake genes. The reductase from the schizokinen-producing alkaliphile Bacillus halodurans was found to cluster with a ferric citrate-hydroxamate uptake system and to catalyze iron release efficiently from Fe[III]-dicitrate, Fe[III]-schizokinen, Fe[III]-aerobactin, and ferrichrome. The gene was hence named fchR for ferric citrate and hydroxamate reductase. The tightly bound [2Fe-2S] cofactor of FchR was identified by UV-visible, EPR, CD spectroscopy, and mass spectrometry. Iron release kinetics were determined with several substrates by using ferredoxin as electron donor. Catalytic efficiencies were strongly enhanced in the presence of an iron-sulfur scaffold protein scavenging the released ferrous iron. Competitive inhibition of FchR was observed with Ga(III)-charged siderophores with K(i) values in the micromolar range. The principal catalytic mechanism was found to couple increasing K(m) and K(D) values of substrate binding with increasing k(cat) values, resulting in high catalytic efficiencies over a wide redox range. Physiologically, a chromosomal fchR deletion led to strongly impaired growth during iron limitation even in the presence of ferric siderophores. Inductively coupled plasma-MS analysis of ΔfchR revealed intracellular iron accumulation, indicating that the ferric substrates were not efficiently metabolized. We further show that FchR can be efficiently inhibited by redox-inert siderophore mimics in vivo, suggesting that substrate-specific ferric siderophore reductases may present future targets for microbial pathogen control.
Figures




Similar articles
-
The siderophore-interacting protein YqjH acts as a ferric reductase in different iron assimilation pathways of Escherichia coli.Biochemistry. 2011 Dec 20;50(50):10951-64. doi: 10.1021/bi201517h. Epub 2011 Nov 18. Biochemistry. 2011. PMID: 22098718
-
FhuF, part of a siderophore-reductase system.Biochemistry. 2004 Feb 10;43(5):1386-92. doi: 10.1021/bi0357661. Biochemistry. 2004. PMID: 14756576
-
Conjuring up a ghost: structural and functional characterization of FhuF, a ferric siderophore reductase from E. coli.J Biol Inorg Chem. 2021 May;26(2-3):313-326. doi: 10.1007/s00775-021-01854-y. Epub 2021 Feb 9. J Biol Inorg Chem. 2021. PMID: 33559753 Free PMC article.
-
[New Drug Discovery Targeting Iron in Bacterial Infectious Diseases].Yakugaku Zasshi. 2024;144(6):633-641. doi: 10.1248/yakushi.23-00197-2. Yakugaku Zasshi. 2024. PMID: 38825472 Review. Japanese.
-
Iron uptake by fungi: contrasted mechanisms with internal or external reduction.Adv Microb Physiol. 2000;43:39-74. doi: 10.1016/s0065-2911(00)43002-x. Adv Microb Physiol. 2000. PMID: 10907554 Review.
Cited by
-
The Effect of Visible Light on Cell Envelope Subproteome during Vibrio harveyi Survival at 20 °C in Seawater.Microorganisms. 2021 Mar 13;9(3):594. doi: 10.3390/microorganisms9030594. Microorganisms. 2021. PMID: 33805730 Free PMC article.
-
Cyanobacterial Siderophores-Physiology, Structure, Biosynthesis, and Applications.Mar Drugs. 2019 May 10;17(5):281. doi: 10.3390/md17050281. Mar Drugs. 2019. PMID: 31083354 Free PMC article. Review.
-
A highly stable plastidic-type ferredoxin-NADP(H) reductase in the pathogenic bacterium Leptospira interrogans.PLoS One. 2011;6(10):e26736. doi: 10.1371/journal.pone.0026736. Epub 2011 Oct 24. PLoS One. 2011. PMID: 22039544 Free PMC article.
-
Ferric iron reductases and their contribution to unicellular ferrous iron uptake.J Inorg Biochem. 2021 May;218:111407. doi: 10.1016/j.jinorgbio.2021.111407. Epub 2021 Feb 25. J Inorg Biochem. 2021. PMID: 33684686 Free PMC article. Review.
-
Not All That Glitters Is Gold: The Paradox of CO-dependent Hydrogenogenesis in Parageobacillus thermoglucosidasius.Front Microbiol. 2021 Dec 9;12:784652. doi: 10.3389/fmicb.2021.784652. eCollection 2021. Front Microbiol. 2021. PMID: 34956151 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

