The oral iron chelator deferiprone protects against iron overload-induced retinal degeneration
- PMID: 21051716
- PMCID: PMC4183363
- DOI: 10.1167/iovs.10-6207
The oral iron chelator deferiprone protects against iron overload-induced retinal degeneration
Abstract
Purpose: Iron-induced oxidative stress may exacerbate age-related macular degeneration (AMD). Ceruloplasmin/Hephaestin double-knockout (DKO) mice with age-dependent retinal iron accumulation and some features of AMD were used to test retinal protection by the oral iron chelator deferiprone (DFP).
Methods: Cultured retinal pigment epithelial (ARPE-19) cells and mice were treated with DFP. Transferrin receptor mRNA (Tfrc), an indicator of iron levels, was quantified by qPCR. In mice, retinal oxidative stress was assessed by mass spectrometry, and degeneration by histology and electroretinography.
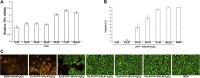
Results: DFP at 60 μM decreased labile iron in ARPE-19 cells, increasing Tfrc and protecting 70% of cells against a lethal dose of H(2)O(2). DFP 1 mg/mL in drinking water increased retinal Tfrc mRNA 2.7-fold after 11 days and also increased transferrin receptor protein. In DKOs, DFP over 8 months decreased retinal iron levels to 72% of untreated mice, diminished retinal oxidative stress to 70% of the untreated level, and markedly ameliorated retinal degeneration. DFP was not retina toxic in wild-type (WT) or DKO mice, as assessed by histology and electroretinography.
Conclusions: Oral DFP was not toxic to the mouse retina. It diminished retinal iron levels and oxidative stress and protected DKO mice against iron overload-induced retinal degeneration. Further testing of DFP for retinal disease involving oxidative stress is warranted.
Figures





References
-
- Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol. 2003;121:1099–1105 - PubMed
-
- Kontoghiorghes GJ, Efstathiou A, Kleanthous M, Michaelides Y, Kolnagou A. Risk/benefit assessment, advantages over other drugs and targeting methods in the use of deferiprone as a pharmaceutical antioxidant in iron loading and non iron loading conditions. Hemoglobin. 2009;33:386–397 - PubMed
-
- Snyder AM, Connor JR. Iron, the substantia nigra and related neurological disorders. Biochim Biophys Acta. 2009;1790:606–614 - PubMed
-
- Sullivan JL. Iron in arterial plaque: modifiable risk factor for atherosclerosis. Biochim Biophys Acta. 2009;1790:718–723 - PubMed
-
- Zacharski LR, Chow BK, Howes PS, et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

