Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: a randomized phase II study
- PMID: 21051752
- PMCID: PMC3052236
- DOI: 10.2215/CJN.05840710
Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: a randomized phase II study
Abstract
Background and objectives: Prolonged use of calcineurin inhibitors (CNIs) in kidney transplant recipients is associated with renal and nonrenal toxicity and an increase in cardiovascular risk factors. Belatacept-based regimens may provide a treatment option for patients who switch from CNI-based maintenance immunosuppression.
Design, setting, participants, & measurements: This is a randomized, open-label Phase II trial in renal transplant patients with stable graft function and receiving a CNI-based regimen. Patients who were ≥6 months but ≤36 months after transplantation were randomized to either switch to belatacept or continue CNI treatment. All patients received background maintenance immunosuppression. The primary end point was the change in calculated GFR (cGFR) from baseline to month 12.
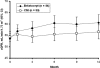
Results: Patients were randomized either to switch to belatacept (n=84) or to remain on a CNI-based regimen (n=89). At month 12, the mean (SD) change from baseline in cGFR was higher in the belatacept group versus the CNI group. Six patients in the belatacept group had acute rejection episodes, all within the first 6 months; all resolved with no allograft loss. By month 12, one patient in the CNI group died with a functioning graft, whereas no patients in the belatacept group had graft loss. The overall safety profile was similar between groups.
Conclusions: The study identifies a potentially safe and feasible method for switching stable renal transplant patients from a cyclosporine- or tacrolimus-based regimen to a belatacept-based regimen, which may allow improved renal function in patients currently treated with CNIs.
Trial registration: ClinicalTrials.gov NCT00402168.
Figures
Similar articles
-
Safety and Efficacy Outcomes 3 Years After Switching to Belatacept From a Calcineurin Inhibitor in Kidney Transplant Recipients: Results From a Phase 2 Randomized Trial.Am J Kidney Dis. 2017 May;69(5):587-594. doi: 10.1053/j.ajkd.2016.09.021. Epub 2016 Nov 23. Am J Kidney Dis. 2017. PMID: 27889299 Clinical Trial.
-
Late conversion from tacrolimus to a belatacept-based immuno-suppression regime in kidney transplant recipients improves renal function, acid-base derangement and mineral-bone metabolism.J Nephrol. 2017 Aug;30(4):607-615. doi: 10.1007/s40620-017-0411-0. Epub 2017 May 24. J Nephrol. 2017. PMID: 28540602
-
Conversion from Calcineurin Inhibitor- to Belatacept-Based Maintenance Immunosuppression in Renal Transplant Recipients: A Randomized Phase 3b Trial.J Am Soc Nephrol. 2021 Dec 1;32(12):3252-3264. doi: 10.1681/ASN.2021050628. Epub 2021 Dec 1. J Am Soc Nephrol. 2021. PMID: 34706967 Free PMC article. Clinical Trial.
-
Treatment strategies in pediatric solid organ transplant recipients with calcineurin inhibitor-induced nephrotoxicity.Pediatr Transplant. 2006 Sep;10(6):721-9. doi: 10.1111/j.1399-3046.2006.00577.x. Pediatr Transplant. 2006. PMID: 16911497 Review.
-
How the development of new biological agents may help minimize immunosuppression in kidney transplantation: the impact of belatacept.Curr Opin Organ Transplant. 2010 Dec;15(6):697-702. doi: 10.1097/MOT.0b013e3283402b5c. Curr Opin Organ Transplant. 2010. PMID: 20930638 Review.
Cited by
-
Disorders of potassium homeostasis after kidney transplantation.World J Transplant. 2024 Sep 18;14(3):95905. doi: 10.5500/wjt.v14.i3.95905. World J Transplant. 2024. PMID: 39295980 Free PMC article. Review.
-
Novel immunosuppressive agents in kidney transplantation.World J Transplant. 2013 Dec 24;3(4):68-77. doi: 10.5500/wjt.v3.i4.68. World J Transplant. 2013. PMID: 24392311 Free PMC article. Review.
-
Immunosuppressive drug therapy.Cold Spring Harb Perspect Med. 2013 Sep 1;3(9):a015487. doi: 10.1101/cshperspect.a015487. Cold Spring Harb Perspect Med. 2013. PMID: 24003247 Free PMC article. Review.
-
T-cell co-stimulatory blockade in kidney transplantation: back to the bench.Kidney Int Suppl (2011). 2011 Aug;1(2):25-30. doi: 10.1038/kisup.2011.8. Kidney Int Suppl (2011). 2011. PMID: 25018899 Free PMC article.
-
Targets of new immunosuppressants in renal transplantation.Kidney Int Suppl (2011). 2011 Aug;1(2):47-51. doi: 10.1038/kisup.2011.12. Kidney Int Suppl (2011). 2011. PMID: 25028624 Free PMC article.
References
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004 - PubMed
-
- Naesens M, Kuypers DR, Sarwal M: Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009 - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 - PubMed
-
- Ducloux D, Motte G, Kribs M, Abdelfatah AB, Bresson-Vautrin C, Rebibou JM, Chalopin JM: Hypertension in renal transplantation: Donor and recipient risk factors. Clin Nephrol 57: 409–413, 2002 - PubMed
-
- Mathis AS, Dave N, Knipp GT, Friedman GS: Drug-related dyslipidemia after renal transplantation. Am J Health Syst Pharm 61: 565–585, 2004 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical