Modulation of CD4+ T-cell activation by CD95 co-stimulation
- PMID: 21052094
- PMCID: PMC3131913
- DOI: 10.1038/cdd.2010.134
Modulation of CD4+ T-cell activation by CD95 co-stimulation
Abstract
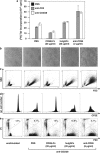
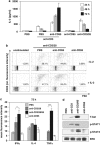
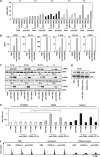
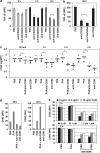
CD95 is a dual-function receptor that exerts pro- or antiapoptotic effects depending on the cellular context, the state of activation, the signal threshold and the mode of ligation. In this study, we report that CD95 engagement modulates TCR/CD3-driven signaling pathways in resting T lymphocytes in a dose-dependent manner. While high doses of immobilized CD95 agonists silence T cells, lower concentrations augment activation and proliferation. We analyzed the co-stimulatory capacity of CD95 in detail in resting human CD4(+) T cells, and demonstrate that low-dose ligand-induced co-internalization of CD95 and TCR/CD3 complexes enables non-apoptotic caspase activation, the prolonged activation of MAP kinases, the upregulation of antiapoptotic proteins associated with apoptosis resistance, and the activation of transcription factors and cell-cycle regulators for the induction of proliferation and cytokine production. We propose that the levels of CD95L on antigen-presenting cells (APCs), neighboring T cells or epithelial cells regulate inhibitory or co-stimulatory CD95 signaling, which in turn is crucial for fine-tuning of primary T-cell activation.
© 2011 Macmillan Publishers Limited
Figures



Similar articles
-
CD95 co-stimulation blocks activation of naive T cells by inhibiting T cell receptor signaling.J Exp Med. 2009 Jun 8;206(6):1379-93. doi: 10.1084/jem.20082363. Epub 2009 Jun 1. J Exp Med. 2009. PMID: 19487421 Free PMC article.
-
Constitutive caspase activation and impaired death-inducing signaling complex formation in CD95-resistant, long-term activated, antigen-specific T cells.J Immunol. 2003 Aug 1;171(3):1172-82. doi: 10.4049/jimmunol.171.3.1172. J Immunol. 2003. PMID: 12874203
-
Lack of activation induced cell death in human T blasts despite CD95L up-regulation: protection from apoptosis by MEK signalling.Immunology. 1999 Dec;98(4):569-75. doi: 10.1046/j.1365-2567.1999.00925.x. Immunology. 1999. PMID: 10594690 Free PMC article.
-
Tyrosine phosphorylation and CD95: a FAScinating switch.Cell Cycle. 2009 Mar 15;8(6):838-42. doi: 10.4161/cc.8.6.7906. Epub 2009 Mar 22. Cell Cycle. 2009. PMID: 19221505 Review.
-
Life in the Fas lane: differential outcomes of Fas signaling.Cell Mol Life Sci. 2013 Nov;70(21):4085-99. doi: 10.1007/s00018-013-1327-z. Epub 2013 Apr 12. Cell Mol Life Sci. 2013. PMID: 23579628 Free PMC article. Review.
Cited by
-
Combination of biological screening in a cellular model of viral latency and virtual screening identifies novel compounds that reactivate HIV-1.J Virol. 2012 Apr;86(7):3795-808. doi: 10.1128/JVI.05972-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258251 Free PMC article.
-
Fas signaling-mediated TH9 cell differentiation favors bowel inflammation and antitumor functions.Nat Commun. 2019 Jul 2;10(1):2924. doi: 10.1038/s41467-019-10889-4. Nat Commun. 2019. PMID: 31266950 Free PMC article.
-
Longitudinal peripheral blood transcriptional analysis of a patient with severe Ebola virus disease.Sci Transl Med. 2017 Apr 12;9(385):eaai9321. doi: 10.1126/scitranslmed.aai9321. Sci Transl Med. 2017. PMID: 28404864 Free PMC article.
-
Fas/FasL Signaling Regulates CD8 Expression During Exposure to Self-Antigens.Front Immunol. 2021 Mar 24;12:635862. doi: 10.3389/fimmu.2021.635862. eCollection 2021. Front Immunol. 2021. PMID: 33841416 Free PMC article.
-
Immune mechanisms and predictive biomarkers related to neoadjuvant immunotherapy response in stage III melanoma.Heliyon. 2024 Jun 11;10(12):e32624. doi: 10.1016/j.heliyon.2024.e32624. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38975149 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

