ING Genes Work as Tumor Suppressor Genes in the Carcinogenesis of Head and Neck Squamous Cell Carcinoma
- PMID: 21052543
- PMCID: PMC2968421
- DOI: 10.1155/2011/963614
ING Genes Work as Tumor Suppressor Genes in the Carcinogenesis of Head and Neck Squamous Cell Carcinoma
Abstract
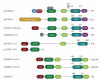
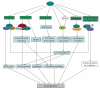
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world. The evolution and progression of HNSCC are considered to result from multiple stepwise alterations of cellular and molecular pathways in squamous epithelium. Recently, inhibitor of growth gene (ING) family consisting of five genes, ING1 to ING5, was identified as a new tumor suppressor gene family that was implicated in the downregulation of cell cycle and chromatin remodeling. In contrast, it has been shown that ING1 and ING2 play an oncogenic role in some cancers, this situation being similar to TGF-β. In HNSCC, the ING family has been reported to be downregulated, and ING translocation from the nucleus to the cytoplasm may be a critical event for carcinogenesis. In this paper, we describe our recent results and briefly summarize current knowledge regarding the biologic functions of ING in HNSCC.
Figures

Similar articles
-
The ING tumor suppressors in cellular senescence and chromatin.Cell Biosci. 2011 Jul 18;1(1):25. doi: 10.1186/2045-3701-1-25. Cell Biosci. 2011. PMID: 21767350 Free PMC article.
-
Alterations in novel candidate tumor suppressor genes, ING1 and ING2 in human lung cancer.Oncol Rep. 2006 Mar;15(3):545-9. Oncol Rep. 2006. PMID: 16465410
-
Downregulation of nuclear ING3 expression and translocalization to cytoplasm promotes tumorigenesis and progression in head and neck squamous cell carcinoma (HNSCC).Histol Histopathol. 2020 Jul;35(7):681-690. doi: 10.14670/HH-18-197. Epub 2019 Dec 30. Histol Histopathol. 2020. PMID: 31886514
-
Focus-ING on DNA Integrity: Implication of ING Proteins in Cell Cycle Regulation and DNA Repair Modulation.Cancers (Basel). 2019 Dec 24;12(1):58. doi: 10.3390/cancers12010058. Cancers (Basel). 2019. PMID: 31878273 Free PMC article. Review.
-
Newly identified tumor suppressor functions of ING proteins.Curr Opin Pharmacol. 2023 Feb;68:102324. doi: 10.1016/j.coph.2022.102324. Epub 2022 Dec 13. Curr Opin Pharmacol. 2023. PMID: 36521226 Review.
Cited by
-
ING3 is associated with increased cell invasion and lethal outcome in ERG-negative prostate cancer patients.Tumour Biol. 2016 Jul;37(7):9731-8. doi: 10.1007/s13277-016-4802-y. Epub 2016 Jan 23. Tumour Biol. 2016. PMID: 26803516
-
Integrated analysis of DNA methylation and mRNA expression profiles to identify key genes involved in the regrowth of clinically non-functioning pituitary adenoma.Aging (Albany NY). 2020 Feb 3;12(3):2408-2427. doi: 10.18632/aging.102751. Epub 2020 Feb 3. Aging (Albany NY). 2020. PMID: 32015217 Free PMC article.
-
Cytoplasmic expression of p33(ING1b) is correlated with tumorigenesis and progression of human esophageal squamous cell carcinoma.Oncol Lett. 2013 Jan;5(1):161-166. doi: 10.3892/ol.2012.983. Epub 2012 Oct 22. Oncol Lett. 2013. PMID: 23255913 Free PMC article.
-
Phosphatidylinositol 5 Phosphate (PI5P): From Behind the Scenes to the Front (Nuclear) Stage.Int J Mol Sci. 2019 Apr 27;20(9):2080. doi: 10.3390/ijms20092080. Int J Mol Sci. 2019. PMID: 31035587 Free PMC article. Review.
-
MicroRNA-9 inhibits growth and invasion of head and neck cancer cells and is a predictive biomarker of response to plerixafor, an inhibitor of its target CXCR4.Mol Oncol. 2018 Dec;12(12):2023-2041. doi: 10.1002/1878-0261.12352. Epub 2018 Oct 25. Mol Oncol. 2018. PMID: 29959873 Free PMC article.
References
-
- Báez A. Genetic and environmental factors in head and neck cancer genesis. Journal of Environmental Science and Health C. 2008;26(2):174–200. - PubMed
-
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of the National Cancer Institute. 2000;92(9):709–720. - PubMed
-
- D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. The New England Journal of Medicine. 2007;356(19):1944–1956. - PubMed
-
- Haddad RI, Shin DM. Recent advances in head and neck cancer. The New England Journal of Medicine. 2008;359(11):1143–1154. - PubMed
-
- Lai S, Batsakis JG, Ordonez NG, Luna MA, Goepfert H, El-Naggar AK. Genotypic and phenotypic alterations of p53 in head and neck squamous cell carcinoma. Oncology Reports. 1995;2(6):1115–1120. - PubMed
LinkOut - more resources
Full Text Sources

