A facile approach toward multicomponent supramolecular structures: selective self-assembly via charge separation
- PMID: 21053935
- PMCID: PMC3016897
- DOI: 10.1021/ja106251f
A facile approach toward multicomponent supramolecular structures: selective self-assembly via charge separation
Abstract
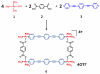
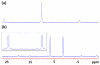
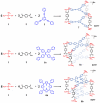
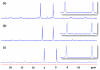
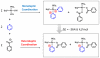
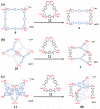
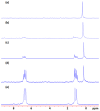
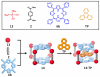
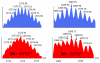
A novel approach toward the construction of multicomponent two-dimensional (2-D) and three-dimensional (3-D) metallosupramolecules is reported. Simply by mixing carboxylate and pyridyl ligands with cis-Pt(PEt(3))(2)(OTf)(2) in a proper ratio, coordination-driven self-assembly occurs, allowing for the selective generation of discrete multicomponent structures via charge separation on the metal centers. Using this method, a variety of 2-D rectangles and 3-D prisms were prepared under mild conditions. Moreover, multicomponent self-assembly can also be achieved by supramolecule-to-supramolecule transformations. The products were characterized by (31)P and (1)H multinuclear NMR spectroscopy, electrospray ionization mass spectrometry, and pulsed-field-gradient spin echo NMR techniques together with computational simulations.
Figures


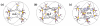



References
-
- Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502.
- Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. - PubMed
- Holliday BJ, Mirkin CA. Angew. Chem., Int. Ed. 2001;40:2022. - PubMed
- Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001:509.
- Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. - PubMed
- Ruben M, Rojo J, Romero-Salguero FJ, Uppadine LH, Lehn J-M. Angew. Chem., Int. Ed. 2004;43:3644. (b) - PubMed
- Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:351. - PubMed
- Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369. - PubMed
- Lukin O, Voegtle F. Angew. Chem., Int. Ed. 2005;44:1456. - PubMed
- Severin K. Chem. Commun. 2006:3859. - PubMed
- Nitschke JR. Acc. Chem. Res. 2007;40:103. - PubMed
- Pitt MA, Johnson DW. Chem. Soc. Rev. 2007;36:1441. - PubMed
- Oliver CG, Ulman PA, Wiester MJ, Mirkin CA. Acc. Chem. Res. 2008;41:1618. - PMC - PubMed
- Parkash MJ, Lah MS. Chem. Commun. 2009:3326. - PubMed
-
- Yang H-B, Das N, Huang F, Hawkridge AM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2006;128:10014. - PubMed
- Yang H-B, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:2120. - PubMed
- Baytekin HT, Sahre M, Rang A, Engeser M, Schulz A, Schalley CA. Small. 2008;4:1823. - PubMed
- Yang H-B, Northrop BH, Zheng Y-R, Ghosh K, Lyndon MM, Muddiman DC, Stang PJ. J. Org. Chem. 2009;74:3524. - PMC - PubMed
- Yang H-B, Northrop BH, Zheng Y-R, Ghosh K, Stang PJ. J. Org. Chem. 2009;74:7067. - PMC - PubMed
- Zheng Y-R, Ghosh K, Yang H-B, Stang PJ. Inorg. Chem. 2010;49:4747. - PMC - PubMed
-
- Pluth MD, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2008;130:6362. - PubMed
- Klosterman JK, Yamauchi Y, Fujita M. Chem. Soc. Rev. 2009;38:1714. - PubMed
- Yamauchia Y, Yoshizawa M, Akita M, Fujita M. Prod. Nat. Acd. Sci. USA. 2009;106:10435. - PMC - PubMed
- Hatakeyama Y, Sawada T, Kawano M, Fujita M. Angew. Chem., Int. Ed. 2009;48:8695. - PubMed
- Pluth MD, Fiedler D, Mugridge JS, Bergman RG, Raymond KN. Prod. Nat. Acd. Sci. USA. 2009;106:10438. - PMC - PubMed
- Mal P, Breiner B, Rissanen K, Nitschke JR. Science. 2009;324:1697. - PubMed
- Sawada T, Fujita M. J. Am. Chem. Soc. 2010;132:7194. - PubMed
-
- Yoshizawa M, Tamura M, Fujita M. Science. 2006;312:251. - PubMed
- Pluth, Michael D, Bergman, Robert G, Raymond, Kenneth N. Science. 2007;316:85. - PubMed
- Pluth MD, Bergman RG, Raymond KN. Acc. Chem. Res. 2009;42:1650. - PubMed
- Yoshizawa M, Klosterman JK, Fujita M. Angew. Chem., Int. Ed. 2009;48:3418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

