Quantitative imaging reveals heterogeneous growth dynamics and treatment-dependent residual tumor distributions in a three-dimensional ovarian cancer model
- PMID: 21054077
- PMCID: PMC2948043
- DOI: 10.1117/1.3483903
Quantitative imaging reveals heterogeneous growth dynamics and treatment-dependent residual tumor distributions in a three-dimensional ovarian cancer model
Abstract
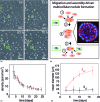
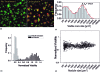
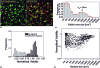
Three-dimensional tumor models have emerged as valuable in vitro research tools, though the power of such systems as quantitative reporters of tumor growth and treatment response has not been adequately explored. We introduce an approach combining a 3-D model of disseminated ovarian cancer with high-throughput processing of image data for quantification of growth characteristics and cytotoxic response. We developed custom MATLAB routines to analyze longitudinally acquired dark-field microscopy images containing thousands of 3-D nodules. These data reveal a reproducible bimodal log-normal size distribution. Growth behavior is driven by migration and assembly, causing an exponential decay in spatial density concomitant with increasing mean size. At day 10, cultures are treated with either carboplatin or photodynamic therapy (PDT). We quantify size-dependent cytotoxic response for each treatment on a nodule by nodule basis using automated segmentation combined with ratiometric batch-processing of calcein and ethidium bromide fluorescence intensity data (indicating live and dead cells, respectively). Both treatments reduce viability, though carboplatin leaves micronodules largely structurally intact with a size distribution similar to untreated cultures. In contrast, PDT treatment disrupts micronodular structure, causing punctate regions of toxicity, shifting the distribution toward smaller sizes, and potentially increasing vulnerability to subsequent chemotherapeutic treatment.
Figures

References
-
- Bissell M. J., Weaver V. M., Lelievre S. A., Wang F., Petersen O. W., and Schmeichel K. L., “Tissue structure, nuclear organization, and gene expression in normal and malignant breast,” Cancer Res. CNREA8 59(7 Suppl.), 1757–1763s, discussion 1763s–1764s (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

