Actin cytoskeleton-dependent Rab GTPase-regulated angiotensin type I receptor lysosomal degradation studied by fluorescence lifetime imaging microscopy
- PMID: 21054097
- PMCID: PMC2966490
- DOI: 10.1117/1.3484751
Actin cytoskeleton-dependent Rab GTPase-regulated angiotensin type I receptor lysosomal degradation studied by fluorescence lifetime imaging microscopy
Abstract
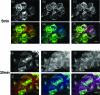
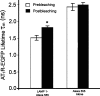
The dynamic regulation of the cellular trafficking of human angiotensin (Ang) type 1 receptor (AT1R) is not well understood. Therefore, we investigated the cellular trafficking of AT1R-enhanced green fluorescent protein (EGFP) (AT1R-EGFP) heterologously expressed in HEK293 cells by determining the change in donor lifetime (AT1R-EGFP) in the presence or absence of acceptor(s) using fluorescence lifetime imaging-fluorescence resonance energy transfer (FRET) microscopy. The average lifetime of AT1R-EGFP in our donor-alone samples was ∼2.33 ns. The basal state lifetime was shortened slightly in the presence of Rab5 (2.01±0.10 ns) or Rab7 (2.11±0.11 ns) labeled with Alexa 555, as the acceptor fluorophore. A 5-min Ang II treatment markedly shortened the lifetime of AT1R-EGFP in the presence of Rab5-Alexa 555 (1.78±0.31 ns) but was affected minimally in the presence of Rab7-Alexa 555 (2.09±0.37 ns). A 30-min Ang II treatment further decreased the AT1R-EGFP lifetime in the presence of both Rab5- and Rab7-Alexa 555. Latrunculin A but not nocodazole pretreatment blocked the ability of Ang II to shorten the AT1R-EGFP lifetime. The occurrence of FRET between AT1R-EGFP (donor) and LAMP1-Alexa 555 (acceptor) with Ang II stimulation was impaired by photobleaching the acceptor. These studies demonstrate that Ang II-induced AT1R lysosomal degradation through its association with LAMP1 is regulated by Rab5/7 via mechanisms that are dependent on intact actin cytoskeletons.
Figures



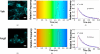



Similar articles
-
Rab7: a key to lysosome biogenesis.Mol Biol Cell. 2000 Feb;11(2):467-80. doi: 10.1091/mbc.11.2.467. Mol Biol Cell. 2000. PMID: 10679007 Free PMC article.
-
Regulation of angiotensin II type 1A receptor intracellular retention, degradation, and recycling by Rab5, Rab7, and Rab11 GTPases.J Biol Chem. 2004 Mar 26;279(13):13110-8. doi: 10.1074/jbc.M313333200. Epub 2004 Jan 7. J Biol Chem. 2004. PMID: 14711821
-
Quantitative comparison of different fluorescent protein couples for fast FRET-FLIM acquisition.Biophys J. 2009 Oct 21;97(8):2368-76. doi: 10.1016/j.bpj.2009.07.044. Biophys J. 2009. PMID: 19843469 Free PMC article.
-
Bivalent angiotensin II suppresses oxidative stress-induced hyper-responsiveness of angiotensin II receptor type I.Eur J Med Chem. 2013 May;63:629-34. doi: 10.1016/j.ejmech.2013.02.041. Epub 2013 Mar 14. Eur J Med Chem. 2013. PMID: 23567951
-
Mechanisms of angiotensin II signaling on cytoskeleton of podocytes.J Mol Med (Berl). 2008 Dec;86(12):1379-94. doi: 10.1007/s00109-008-0399-y. Epub 2008 Sep 5. J Mol Med (Berl). 2008. PMID: 18773185
Cited by
-
Monitoring Keap1-Nrf2 interactions in single live cells.Biotechnol Adv. 2014 Nov 1;32(6):1133-44. doi: 10.1016/j.biotechadv.2014.03.004. Epub 2014 Mar 25. Biotechnol Adv. 2014. PMID: 24681086 Free PMC article. Review.
-
S1P1 receptor phosphorylation, internalization, and interaction with Rab proteins: effects of sphingosine 1-phosphate, FTY720-P, phorbol esters, and paroxetine.Biosci Rep. 2018 Dec 11;38(6):BSR20181612. doi: 10.1042/BSR20181612. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 30366961 Free PMC article.
-
The Pseudo signal peptide of the corticotropin-releasing factor receptor type 2A prevents receptor oligomerization.J Biol Chem. 2012 Aug 3;287(32):27265-74. doi: 10.1074/jbc.M112.360594. Epub 2012 Jun 11. J Biol Chem. 2012. PMID: 22689579 Free PMC article.
-
Investigating protein-protein interactions in living cells using fluorescence lifetime imaging microscopy.Nat Protoc. 2011 Aug 11;6(9):1324-40. doi: 10.1038/nprot.2011.364. Nat Protoc. 2011. PMID: 21886099 Free PMC article.
-
Förster resonance energy transfer microscopy and spectroscopy for localizing protein-protein interactions in living cells.Cytometry A. 2013 Sep;83(9):780-93. doi: 10.1002/cyto.a.22321. Epub 2013 Jun 27. Cytometry A. 2013. PMID: 23813736 Free PMC article. Review.
References
-
- de Gasparo M., Catt K. J., Inagami T., Wright J. W., and Unger T., “International union of pharmacology. XXIII. The angiotensin II receptors,” Pharmacol. Rev. ZZZZZZ 52, 415–472 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

