Mesenchymal cells of the intestinal lamina propria
- PMID: 21054163
- PMCID: PMC3754809
- DOI: 10.1146/annurev.physiol.70.113006.100646
Mesenchymal cells of the intestinal lamina propria
Abstract
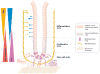
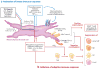
The mesenchymal elements of the intestinal lamina propria reviewed here are the myofibroblasts, fibroblasts, mural cells (pericytes) of the vasculature, bone marrow-derived stromal stem cells, smooth muscle of the muscularis mucosae, and smooth muscle surrounding the lymphatic lacteals. These cells share similar marker molecules, origins, and coordinated biological functions previously ascribed solely to subepithelial myofibroblasts. We review the functional anatomy of intestinal mesenchymal cells and describe what is known about their origin in the embryo and their replacement in adults. As part of their putative role in intestinal mucosal morphogenesis, we consider the intestinal stem cell niche. Lastly, we review emerging information about myofibroblasts as nonprofessional immune cells that may be important as an alarm system for the gut and as a participant in peripheral immune tolerance.
Figures


References
LITERATURE CITED
-
- Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors. I Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol. 2005;289:2–7. - PubMed
-
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol. 1999;277:183–201. - PubMed
-
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I Paracrine cells important in health and disease. Am J Physiol Cell Physiol. 1999;277:1–9. - PubMed
-
- Simon-Assmann P, Bolcato-Bellemin AL, Klein A, Kedinger M. Tissue recombinants to study extracellular matrix targeting to basement membranes. Methods Mol Biol. 2009;522:309–18. - PubMed
RELATED RESOURCES
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. - PubMed
-
- Roberts DJ. Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn. 2000;219:109–20. - PubMed
-
- McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–91. - PubMed
-
- Rubin DC. Intestinal morphogenesis. Curr Opin Gastroenterology. 2007;23:111–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

