Purine receptor-mediated endocannabinoid production and retrograde synaptic signalling in the cerebellar cortex
- PMID: 21054344
- PMCID: PMC3042206
- DOI: 10.1111/j.1476-5381.2010.01106.x
Purine receptor-mediated endocannabinoid production and retrograde synaptic signalling in the cerebellar cortex
Abstract
Background and purpose: Presynaptic CB₁ cannabinoid receptors can be activated by endogenous cannabinoids (endocannabinoids) synthesized by postsynaptic neurones. The hypothesis of the present work was that activation of calcium-permeable transmitter-gated ion channels in postsynaptic neurones, specifically of P2X purine receptors, can lead to endocannabinoid production and retrograde synaptic signalling.
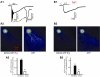
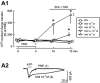
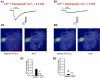
Experimental approach: GABAergic inhibitory postsynaptic currents (IPSCs) were recorded with patch-clamp techniques in Purkinje cells in mouse cerebellar slices. Purine receptors on Purkinje cells were activated by pressure ejection of ATP from a pipette.
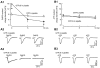
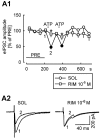
Key results: ATP evoked an inward current in Purkinje cells, most likely due to P2X receptor activation. The ATP-evoked currents were accompanied by currents via voltage-gated calcium channels. ATP suppressed electrical stimulation-evoked IPSCs and miniature IPSCs (mIPSCs) recorded in the presence of tetrodotoxin, and these effects were prevented by the CB₁ antagonist rimonabant and the calcium chelator BAPTA (applied into the Purkinje cell). ATP also suppressed mIPSCs when voltage-gated calcium channels were blocked by cadmium, and intracellular calcium stores were depleted by thapsigargin. However, ATP failed to suppress mIPSCs when the extracellular calcium concentration was zero.
Conclusions and implications: ATP elicits CB₁ receptor-dependent retrograde synaptic suppression, which is probably mediated by an endocannabinod released by the postsynaptic neurone. An increase in intracellular calcium concentration in the postsynaptic neurone is necessary for this retrograde signalling. We propose that ATP increases the calcium concentration by two mechanisms: calcium enters into the neurone via the P2X receptor ion channel and the ATP-evoked depolarization triggers voltage-gated calcium channels.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

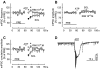
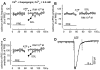

Similar articles
-
Endocannabinoid-mediated synaptically evoked suppression of GABAergic transmission in the cerebellar cortex.Neuroscience. 2010 Sep 1;169(3):1268-78. doi: 10.1016/j.neuroscience.2010.05.036. Epub 2010 May 27. Neuroscience. 2010. PMID: 20553815
-
Depolarizing GABAergic synaptic input triggers endocannabinoid-mediated retrograde synaptic signaling.Synapse. 2009 Aug;63(8):643-52. doi: 10.1002/syn.20641. Synapse. 2009. PMID: 19347961
-
The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells.J Neurosci. 2002 Mar 1;22(5):1690-7. doi: 10.1523/JNEUROSCI.22-05-01690.2002. J Neurosci. 2002. PMID: 11880498 Free PMC article.
-
Retrograde endocannabinoid signaling in the cerebellar cortex.Cerebellum. 2006;5(2):134-45. doi: 10.1080/14734220600791477. Cerebellum. 2006. PMID: 16818388 Review.
-
Cerebellar endocannabinoids: retrograde signaling from purkinje cells.Cerebellum. 2015 Jun;14(3):341-53. doi: 10.1007/s12311-014-0629-5. Cerebellum. 2015. PMID: 25520276 Review.
Cited by
-
Cannabinoid Receptors Modulate Neuronal Morphology and AnkyrinG Density at the Axon Initial Segment.Front Cell Neurosci. 2017 Jan 25;11:5. doi: 10.3389/fncel.2017.00005. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28179879 Free PMC article.
-
Exogenous and endogenous cannabinoids suppress inhibitory neurotransmission in the human neocortex.Neuropsychopharmacology. 2012 Apr;37(5):1104-14. doi: 10.1038/npp.2011.262. Epub 2011 Nov 2. Neuropsychopharmacology. 2012. PMID: 22048459 Free PMC article.
-
Plasticity of mouse enteric synapses mediated through endocannabinoid and purinergic signaling.Neurogastroenterol Motil. 2012 Mar;24(3):e113-24. doi: 10.1111/j.1365-2982.2011.01860.x. Epub 2012 Jan 11. Neurogastroenterol Motil. 2012. PMID: 22235973 Free PMC article.
-
Interaction between cannabinoid and nucleotide systems as a new mechanism of signaling in retinal cell death.Neural Regen Res. 2019 Dec;14(12):2093-2094. doi: 10.4103/1673-5374.262585. Neural Regen Res. 2019. PMID: 31397346 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

