Urine concentrating mechanism in the inner medulla of the mammalian kidney: role of three-dimensional architecture
- PMID: 21054810
- PMCID: PMC3807677
- DOI: 10.1111/j.1748-1716.2010.02214.x
Urine concentrating mechanism in the inner medulla of the mammalian kidney: role of three-dimensional architecture
Abstract
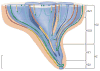
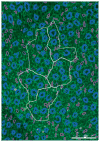
The urine concentrating mechanism in the mammalian renal inner medulla (IM) is not understood, although it is generally considered to involve countercurrent flows in tubules and blood vessels. A possible role for the three-dimensional relationships of these tubules and vessels in the concentrating process is suggested by recent reconstructions from serial sections labelled with antibodies to tubular and vascular proteins and mathematical models based on these studies. The reconstructions revealed that the lower 60% of each descending thin limb (DTL) of Henle's loops lacks water channels (aquaporin-1) and osmotic water permeability and ascending thin limbs (ATLs) begin with a prebend segment of constant length. In the outer zone of the IM (i) clusters of coalescing collecting ducts (CDs) form organizing motif for loops of Henle and vasa recta; (ii) DTLs and descending vasa recta (DVR) are arrayed outside CD clusters, whereas ATLs and ascending vasa recta (AVR) are uniformly distributed inside and outside clusters; (iii) within CD clusters, interstitial nodal spaces are formed by a CD on one side, AVR on two sides, and an ATL on the fourth side. These spaces may function as mixing chambers for urea from CDs and NaCl from ATLs. In the inner zone of the IM, cluster organization disappears and half of Henle's loops have broad lateral bends wrapped around terminal CDs. Mathematical models based on these findings and involving solute mixing in the interstitial spaces can produce urine slightly more concentrated than that of a moderately antidiuretic rat but no higher.
© 2010 The Authors. Acta Physiologica © 2010 Scandinavian Physiological Society.
Conflict of interest statement
None.
Figures














Similar articles
-
Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla.Am J Physiol Renal Physiol. 2008 Nov;295(5):F1271-85. doi: 10.1152/ajprenal.90252.2008. Epub 2008 May 21. Am J Physiol Renal Physiol. 2008. PMID: 18495796 Free PMC article. Review.
-
Quantitative analysis of functional reconstructions reveals lateral and axial zonation in the renal inner medulla.Am J Physiol Renal Physiol. 2008 Jun;294(6):F1306-14. doi: 10.1152/ajprenal.00068.2008. Epub 2008 Apr 16. Am J Physiol Renal Physiol. 2008. PMID: 18417543
-
Three-dimensional architecture of inner medullary vasa recta.Am J Physiol Renal Physiol. 2006 Jun;290(6):F1355-66. doi: 10.1152/ajprenal.00481.2005. Epub 2005 Dec 27. Am J Physiol Renal Physiol. 2006. PMID: 16380456
-
Three-dimensional architecture of collecting ducts, loops of Henle, and blood vessels in the renal papilla.Am J Physiol Renal Physiol. 2007 Sep;293(3):F696-704. doi: 10.1152/ajprenal.00231.2007. Epub 2007 Jul 3. Am J Physiol Renal Physiol. 2007. PMID: 17609288
-
Structure and concentrating ability of the mammalian kidney: correlations with habitat.Am J Physiol. 1996 Jul;271(1 Pt 2):R157-79. doi: 10.1152/ajpregu.1996.271.1.R157. Am J Physiol. 1996. PMID: 8760217 Review.
Cited by
-
Isolated interstitial nodal spaces may facilitate preferential solute and fluid mixing in the rat renal inner medulla.Am J Physiol Renal Physiol. 2012 Apr 1;302(7):F830-9. doi: 10.1152/ajprenal.00539.2011. Epub 2011 Dec 7. Am J Physiol Renal Physiol. 2012. PMID: 22160770 Free PMC article.
-
The importance of the thick ascending limb of Henle's loop in renal physiology and pathophysiology.Int J Nephrol Renovasc Dis. 2018 Feb 15;11:81-92. doi: 10.2147/IJNRD.S154000. eCollection 2018. Int J Nephrol Renovasc Dis. 2018. PMID: 29497325 Free PMC article. Review.
-
Claudin 19 Is Regulated by Extracellular Osmolality in Rat Kidney Inner Medullary Collecting Duct Cells.Int J Mol Sci. 2019 Sep 7;20(18):4401. doi: 10.3390/ijms20184401. Int J Mol Sci. 2019. PMID: 31500238 Free PMC article.
-
Intraglomerular crosstalk elaborately regulates podocyte injury and repair in diabetic patients: insights from a 3D multiscale modeling study.Oncotarget. 2016 Nov 8;7(45):73130-73146. doi: 10.18632/oncotarget.12233. Oncotarget. 2016. PMID: 27683034 Free PMC article.
-
Sex and circadian regulation of metabolic demands in the rat kidney: A modeling analysis.PLoS One. 2024 Jul 17;19(7):e0293419. doi: 10.1371/journal.pone.0293419. eCollection 2024. PLoS One. 2024. PMID: 39018272 Free PMC article.
References
-
- Beck F, Dorge A, Rick R, Thurau K. Osmoregulation of renal papillary cells. Pflugers Arch. 1985;405:S28–S32. - PubMed
-
- Bulger RE, Trump BF. Fine structure of the rat renal papilla. Am J Anat. 1966;118:685–722. - PubMed
-
- Burg MB, Green N. Function of thick ascending limb of Henle’s loop. Am J Physiol. 1973;224:659–668. - PubMed
-
- Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: segmentation and osmotic water permeability. Am J Physiol Renal, Fluid Electrolyte Physiol. 1992;263:F417–F426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

