Prehospital randomised assessment of a mechanical compression device in cardiac arrest (PaRAMeDIC) trial protocol
- PMID: 21054860
- PMCID: PMC2993645
- DOI: 10.1186/1757-7241-18-58
Prehospital randomised assessment of a mechanical compression device in cardiac arrest (PaRAMeDIC) trial protocol
Abstract
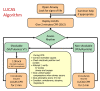
Background: Survival after out-of-hospital cardiac arrest is closely linked to the quality of CPR, but in real life, resuscitation during prehospital care and ambulance transport is often suboptimal. Mechanical chest compression devices deliver consistent chest compressions, are not prone to fatigue and could potentially overcome some of the limitations of manual chest compression. However, there is no high-quality evidence that they improve clinical outcomes, or that they are cost effective. The Prehospital Randomised Assessment of a Mechanical Compression Device In Cardiac Arrest (PARAMEDIC) trial is a pragmatic cluster randomised study of the LUCAS-2 device in adult patients with non-traumatic out-of-hospital cardiac arrest.
Methods/design: The primary objective of this trial is to evaluate the effect of chest compression using LUCAS-2 on mortality at 30 days post out-of-hospital cardiac arrest, compared with manual chest compression. Secondary objectives of the study are to evaluate the effects of LUCAS-2 on survival to 12 months, cognitive and quality of life outcomes and cost-effectiveness.
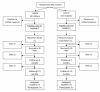
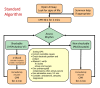
Methods: Ambulance service vehicles will be randomised to either manual compression (control) or LUCAS arms. Adult patients in out-of-hospital cardiac arrest, attended by a trial vehicle will be eligible for inclusion. Patients with traumatic cardiac arrest or who are pregnant will be excluded. The trial will recruit approximately 4000 patients from England, Wales and Scotland. A waiver of initial consent has been approved by the Research Ethics Committees. Consent will be sought from survivors for participation in the follow-up phase.
Conclusion: The trial will assess the clinical and cost effectiveness of the LUCAS-2 mechanical chest compression device.
Trial registration: The trial is registered on the International Standard Randomised Controlled Trial Number Registry (ISRCTN08233942).
Figures
References
-
- London Ambulance Service Cardiac Arrest Annual Report 2008/9. http://www.londonambulance.nhs.uk/about_us/idoc.ashx?docid=498ba02b-466b...
-
- Ambulance Service Association and Joint Royal College Ambulance Liaison Committee. National Cardiac Arrest Audit Report. 2006.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical