Cost analysis of centralized viral load testing for antiretroviral therapy monitoring in Nicaragua, a low-HIV prevalence, low-resource setting
- PMID: 21054866
- PMCID: PMC2992476
- DOI: 10.1186/1758-2652-13-43
Cost analysis of centralized viral load testing for antiretroviral therapy monitoring in Nicaragua, a low-HIV prevalence, low-resource setting
Abstract
Background: HIV viral load testing as a component of antiretroviral therapy monitoring is costly. Understanding the full costs and the major sources of inefficiency associated with viral load testing is critical for optimizing the systems and technologies that support the testing process. The objective of our study was to estimate the costs associated with viral load testing performed for antiretroviral therapy monitoring to both patients and the public healthcare system in a low-HIV prevalence, low-resource country.
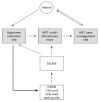
Methods: A detailed cost analysis was performed to understand the costs involved in each step of performing a viral load test in Nicaragua, from initial specimen collection to communication of the test results to each patient's healthcare provider. Data were compiled and cross referenced from multiple information sources: laboratory records, regional surveillance centre records, and scheduled interviews with the key healthcare providers responsible for HIV patient care in five regions of the country.
Results: The total average cost of performing a viral load test in Nicaragua varied by region, ranging from US$99.01 to US$124.58, the majority of which was at the laboratory level: $88.73 to $97.15 per specimen, depending on batch size. The average cost to clinics at which specimens were collected ranged from $3.31 to $20.92, depending on the region. The average cost per patient for transportation, food, lodging and lost income ranged from $3.70 to $14.93.
Conclusions: The quantitative viral load test remains the single most expensive component of the process. For the patient, the distance of his or her residence from the specimen collection site is a large determinant of cost. Importantly, the efficiency of results reporting has a large impact on the cost per result delivered to the clinician and utility of the result for patient monitoring. Detailed cost analysis can identify opportunities for removing barriers to effective antiretroviral therapy monitoring programmes in limited-resource countries with low HIV prevalence.
Figures


Similar articles
-
On the front line of HIV virological monitoring: barriers and facilitators from a provider perspective in resource-limited settings.AIDS Care. 2016;28(1):1-10. doi: 10.1080/09540121.2015.1058896. Epub 2015 Aug 17. AIDS Care. 2016. PMID: 26278724 Free PMC article.
-
[Optimizing resources to reduce costs to determine HIV viral load in limited resources settings].Biomedica. 2017 Dec 1;37(4):460-465. doi: 10.7705/biomedica.v37i4.3318. Biomedica. 2017. PMID: 29373766 Spanish.
-
Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models.Lancet Glob Health. 2014 Jan;2(1):e35-43. doi: 10.1016/S2214-109X(13)70048-2. Epub 2013 Dec 10. Lancet Glob Health. 2014. PMID: 25104633 Free PMC article.
-
The impact of disease stage on direct medical costs of HIV management: a review of the international literature.Pharmacoeconomics. 2010;28 Suppl 1:35-47. doi: 10.2165/11587430-000000000-00000. Pharmacoeconomics. 2010. PMID: 21182342 Review.
-
The case for viral load testing in adolescents in resource-limited settings.J Int AIDS Soc. 2017 Nov;20 Suppl 7(Suppl 7):e25002. doi: 10.1002/jia2.25002. J Int AIDS Soc. 2017. PMID: 29171180 Free PMC article. Review.
Cited by
-
Cost-Effectiveness of Improving Health Care to People with HIV in Nicaragua.Nurs Res Pract. 2014;2014:232046. doi: 10.1155/2014/232046. Epub 2014 May 25. Nurs Res Pract. 2014. PMID: 24977038 Free PMC article.
-
A pilot model of centralized anti-HIV-1 drug resistance testing with decentralized treatment in resource-limited settings.Glob Health Med. 2025 Jun 30;7(3):252-259. doi: 10.35772/ghm.2025.01045. Glob Health Med. 2025. PMID: 40606532 Free PMC article.
-
Perspectives on introduction and implementation of new point-of-care diagnostic tests.J Infect Dis. 2012 May 15;205 Suppl 2(Suppl 2):S181-90. doi: 10.1093/infdis/jis203. Epub 2012 Mar 7. J Infect Dis. 2012. PMID: 22402038 Free PMC article.
-
Evaluation of the performance of a multiplex reverse transcription polymerase chain reaction kit as a potential diagnostic and surveillance kit for rotavirus in Kenya.Trop Dis Travel Med Vaccines. 2019 Jul 15;5:12. doi: 10.1186/s40794-019-0087-7. eCollection 2019. Trop Dis Travel Med Vaccines. 2019. PMID: 31346474 Free PMC article.
-
Progress in the development of paper-based diagnostics for low-resource point-of-care settings.Bioanalysis. 2013 Nov;5(22):2821-36. doi: 10.4155/bio.13.243. Bioanalysis. 2013. PMID: 24256361 Free PMC article. Review.
References
-
- Kimmel AD, Weinstein MC, Anglaret X, Goldie SJ, Losina E, Yazdanpanah Y, Messou E, Cotich KL, Walensky RP, Freedberg KA. Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr. 2010;13(3):258–268. doi: 10.1097/QAI.0b013e3181d0db97. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

