Nipah virus infection and glycoprotein targeting in endothelial cells
- PMID: 21054904
- PMCID: PMC2991316
- DOI: 10.1186/1743-422X-7-305
Nipah virus infection and glycoprotein targeting in endothelial cells
Abstract
Background: The highly pathogenic Nipah virus (NiV) causes fatal respiratory and brain infections in animals and humans. The major hallmark of the infection is a systemic endothelial infection, predominantly in the CNS. Infection of brain endothelial cells allows the virus to overcome the blood-brain-barrier (BBB) and to subsequently infect the brain parenchyma. However, the mechanisms of NiV replication in endothelial cells are poorly elucidated. We have shown recently that the bipolar or basolateral expression of the NiV surface glycoproteins F and G in polarized epithelial cell layers is involved in lateral virus spread via cell-to-cell fusion and that correct sorting depends on tyrosine-dependent targeting signals in the cytoplasmic tails of the glycoproteins. Since endothelial cells share many characteristics with epithelial cells in terms of polarization and protein sorting, we wanted to elucidate the role of the NiV glycoprotein targeting signals in endothelial cells.
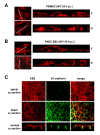
Results: As observed in vivo, NiV infection of endothelial cells induced syncytia formation. The further finding that infection increased the transendothelial permeability supports the idea of spread of infection via cell-to-cell fusion and endothelial cell damage as a mechanism to overcome the BBB. We then revealed that both glycoproteins are expressed at lateral cell junctions (bipolar), not only in NiV-infected primary endothelial cells but also upon stable expression in immortalized endothelial cells. Interestingly, mutation of tyrosines 525 and 542/543 in the cytoplasmic tail of the F protein led to an apical redistribution of the protein in endothelial cells whereas tyrosine mutations in the G protein had no effect at all. This fully contrasts the previous results in epithelial cells where tyrosine 525 in the F, and tyrosines 28/29 in the G protein were required for correct targeting.
Conclusion: We conclude that the NiV glycoprotein distribution is responsible for lateral virus spread in both, epithelial and endothelial cell monolayers. However, the prerequisites for correct protein targeting differ markedly in the two polarized cell types.
Figures


Similar articles
-
Tyrosine residues in the cytoplasmic domains affect sorting and fusion activity of the Nipah virus glycoproteins in polarized epithelial cells.J Virol. 2010 Aug;84(15):7634-41. doi: 10.1128/JVI.02576-09. Epub 2010 May 19. J Virol. 2010. PMID: 20484517 Free PMC article.
-
Timing of galectin-1 exposure differentially modulates Nipah virus entry and syncytium formation in endothelial cells.J Virol. 2015 Mar;89(5):2520-9. doi: 10.1128/JVI.02435-14. Epub 2014 Dec 10. J Virol. 2015. PMID: 25505064 Free PMC article.
-
Nipah and Hendra Virus Glycoproteins Induce Comparable Homologous but Distinct Heterologous Fusion Phenotypes.J Virol. 2019 Jun 14;93(13):e00577-19. doi: 10.1128/JVI.00577-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30971473 Free PMC article.
-
Molecular characteristics of the Nipah virus glycoproteins.Ann N Y Acad Sci. 2007 Apr;1102:39-50. doi: 10.1196/annals.1408.003. Ann N Y Acad Sci. 2007. PMID: 17470910 Review.
-
Envelope-receptor interactions in Nipah virus pathobiology.Ann N Y Acad Sci. 2007 Apr;1102(1):51-65. doi: 10.1196/annals.1408.004. Ann N Y Acad Sci. 2007. PMID: 17470911 Free PMC article. Review.
Cited by
-
Nipah virus infects specific subsets of porcine peripheral blood mononuclear cells.PLoS One. 2012;7(1):e30855. doi: 10.1371/journal.pone.0030855. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303463 Free PMC article.
-
Coronavirus entry and release in polarized epithelial cells: a review.Rev Med Virol. 2014 Sep;24(5):308-15. doi: 10.1002/rmv.1792. Epub 2014 Apr 16. Rev Med Virol. 2014. PMID: 24737708 Free PMC article. Review.
-
Apicobasal polarity of brain endothelial cells.J Cereb Blood Flow Metab. 2016 Feb;36(2):340-62. doi: 10.1177/0271678X15608644. Epub 2015 Oct 6. J Cereb Blood Flow Metab. 2016. PMID: 26661193 Free PMC article. Review.
-
Co-assembly of viral envelope glycoproteins regulates their polarized sorting in neurons.PLoS Pathog. 2014 May 15;10(5):e1004107. doi: 10.1371/journal.ppat.1004107. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24831812 Free PMC article.
-
A key region of molecular specificity orchestrates unique ephrin-B1 utilization by Cedar virus.Life Sci Alliance. 2019 Dec 20;3(1):e201900578. doi: 10.26508/lsa.201900578. Print 2020 Jan. Life Sci Alliance. 2019. PMID: 31862858 Free PMC article.
References
-
- Mohd Nor MN, Gan CH, Ong BL. Nipah virus infection of pigs in peninsular Malaysia. Rev Sci Tech. 2000;19:160–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

