Characterization and functionality of proliferative human Sertoli cells
- PMID: 21054948
- PMCID: PMC4096632
- DOI: 10.3727/096368910X536563
Characterization and functionality of proliferative human Sertoli cells
Abstract
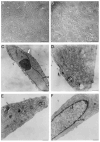
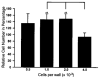
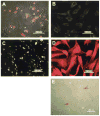
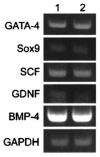
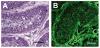
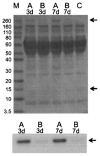
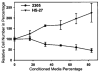
It has long been thought that mammalian Sertoli cells are terminally differentiated and nondividing postpuberty. For most previous in vitro studies immature rodent testes have been the source of Sertoli cells and these have shown little proliferative ability when cultured. We have isolated and characterized Sertoli cells from human cadaveric testes from seven donors ranging from 12 to 36 years of age. The cells proliferated readily in vitro under the optimized conditions used with a doubling time of approximately 4 days. Nuclear 5-ethynyl-2'-deoxyuridine (EdU) incorporation confirmed that dividing cells represented the majority of the population. Classical Sertoli cell ultrastructural features, lipid droplet accumulation, and immunoexpression of GATA-4, Sox9, and the FSH receptor (FSHr) were observed by electron and fluorescence microscopy, respectively. Flow cytometry revealed the expression of GATA-4 and Sox9 by more than 99% of the cells, and abundant expression of a number of markers indicative of multipotent mesenchymal cells. Low detection of endogenous alkaline phosphatase activity after passaging showed that few peritubular myoid cells were present. GATA-4 and SOX9 expression were confirmed by reverse transcription polymerase chain reaction (RT-PCR), along with expression of stem cell factor (SCF), glial cell line-derived neurotrophic factor (GDNF), and bone morphogenic protein 4 (BMP4). Tight junctions were formed by Sertoli cells plated on transwell inserts coated with fibronectin as revealed by increased transepithelial electrical resistance (TER) and polarized secretion of the immunoregulatory protein, galectin-1. These primary Sertoli cell populations could be expanded dramatically in vitro and could be cryopreserved. The results show that functional human Sertoli cells can be propagated in vitro from testicular cells isolated from adult testis. The proliferative human Sertoli cells should have important applications in studying infertility, reproductive toxicology, testicular cancer, and spermatogenesis, and due to their unique biological properties potentially could be useful in cell therapy.
Figures

Similar articles
-
Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, Raman spectrum and biochemical phenotype.Hum Reprod. 2013 Jul;28(7):1863-73. doi: 10.1093/humrep/det068. Epub 2013 Mar 15. Hum Reprod. 2013. PMID: 23503944
-
Long-term culture and analysis of cashmere goat Sertoli cells.In Vitro Cell Dev Biol Anim. 2014 Dec;50(10):918-25. doi: 10.1007/s11626-013-9648-7. Epub 2014 Aug 28. In Vitro Cell Dev Biol Anim. 2014. PMID: 25164184
-
NODAL secreted by male germ cells regulates the proliferation and function of human Sertoli cells from obstructive azoospermia and nonobstructive azoospermia patients.Asian J Androl. 2015 Nov-Dec;17(6):996-1005. doi: 10.4103/1008-682X.159722. Asian J Androl. 2015. PMID: 26289399 Free PMC article.
-
Regulation of GDNF expression in Sertoli cells.Reproduction. 2019 Mar;157(3):R95-R107. doi: 10.1530/REP-18-0239. Reproduction. 2019. PMID: 30620720 Free PMC article. Review.
-
Characterization of rodent Sertoli cell primary cultures.Mol Reprod Dev. 2020 Aug;87(8):857-870. doi: 10.1002/mrd.23402. Epub 2020 Aug 2. Mol Reprod Dev. 2020. PMID: 32743879 Free PMC article. Review.
Cited by
-
CAMSAP2 Is a Microtubule Minus-End Targeting Protein That Regulates BTB Dynamics Through Cytoskeletal Organization.Endocrinology. 2019 Jun 1;160(6):1448-1467. doi: 10.1210/en.2018-01097. Endocrinology. 2019. PMID: 30994903 Free PMC article.
-
Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis?Semin Cell Dev Biol. 2016 Nov;59:141-156. doi: 10.1016/j.semcdb.2016.01.003. Epub 2016 Jan 15. Semin Cell Dev Biol. 2016. PMID: 26779951 Free PMC article. Review.
-
In vitro generation of Sertoli-like and haploid spermatid-like cells from human umbilical cord perivascular cells.Stem Cell Res Ther. 2017 Feb 15;8(1):37. doi: 10.1186/s13287-017-0491-8. Stem Cell Res Ther. 2017. PMID: 28202061 Free PMC article.
-
Environmental toxicants perturb human Sertoli cell adhesive function via changes in F-actin organization mediated by actin regulatory proteins.Hum Reprod. 2014 Jun;29(6):1279-91. doi: 10.1093/humrep/deu011. Epub 2014 Feb 13. Hum Reprod. 2014. PMID: 24532171 Free PMC article.
-
Reprograming skin fibroblasts into Sertoli cells: a patient-specific tool to understand effects of genetic variants on gonadal development.Biol Sex Differ. 2024 Mar 22;15(1):24. doi: 10.1186/s13293-024-00599-y. Biol Sex Differ. 2024. PMID: 38520033 Free PMC article.
References
-
- Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, de Rooij DG. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod. 2009;80:1084–1091. - PubMed
-
- Anthony C, Skinner MK. Cytochemical and biochemical characterization of testicular peritubular myoid cells. Biol Reprod. 1989;40:811–823. - PubMed
-
- Benton G, George J, Kleinman HK, Arnaoutova IP. Advancing science and technology via 3D culture on basement membrane matrix. J Cell Physiol. 2009;221:18–25. - PubMed
-
- Bockers TM, Nieschlag E, Kreutz MR, Bergmann M. Localization of follicle-stimulating hormone (FSH) immunoreactivity and hormone receptor mRNA in testicular tissue of infertile men. Cell Tissue Res. 1994;278:595–600. - PubMed
-
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

