Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study
- PMID: 21055739
- PMCID: PMC4180231
- DOI: 10.1016/j.fertnstert.2010.09.059
Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study
Abstract
Objective: To evaluate the efficacy and tolerability of the P receptor modulator CDB-2914 (Ulipristal, CDB).
Design: Randomized, placebo-controlled double-blind clinical trial.
Setting: Clinical research center.
Patient(s): Premenopausal women with symptomatic uterine fibroids.
Intervention(s): Once-daily oral CDB (10 or 20 mg) or placebo (PLC) for 12 weeks (treatment 1). A second 3-month treatment with CDB (treatment 2) was offered. A computer-generated blocked randomization was used.
Main outcome measure(s): Magnetic resonance imaging (MRI)-determined total fibroid volume (TFV) change was the primary outcome; amenorrhea and quality of life (QOL) were secondary end points.
Result(s): Treatment 1 TFV increased 7% in the PLC group, but decreased 17% and 24% in the CDB10 and CDB20 groups. The TFV decreased further in treatment 2 (-11%). Amenorrhea occurred in 20/26 women taking CDB and none on PLC. Ovulation resumed after CDB. Hemoglobin improved only with CDB (11.9 ± 1.5 to 12.9 ± 1.0 g/dL) as did the Fibroid QOL Questionnaire symptom severity, energy/mood, and concern subscores, and overall QOL scores. The CDB was well tolerated, with no serious adverse events. Adverse events were unchanged during treatments.
Conclusion(s): Administration of CDB-2914 for 3-6 months controls bleeding, reduces fibroid size, and improves QOL.
Trial registration: ClinicalTrials.gov NCT00290251.
Published by Elsevier Inc.
Figures
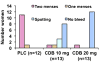

References
-
- Mauskopf J, Flynn M, Thieda P, Spalding J, Duchane J. The economic impact of uterine fibroids in the United States: a summary of published estimates. J Women's Health. 2005;14:692–703. 2002. - PubMed
-
- Williams VS, Jones G, Mauskopf J, Spalding J, DuChane J. Uterine fibroids: a review of health-related quality of life assessment. J Women's Health. 2006;15:818–29. 2002. - PubMed
-
- Bucek RA, Puchner S, Lammer J. Mid- and long-term quality-of-life assessment in patients undergoing uterine fibroid embolization. AJR. 2006;186:877–82. - PubMed
-
- Hartmann KE, Birnbaum H, Ben-Hamadi R, Wu EQ, Farrell MH, Spalding J, et al. Annual costs associated with diagnosis of uterine leiomyomata. Obstet Gynecol. 2006;108:930–7. - PubMed
-
- Lerner D, Mirza F, Chang H, Renzulli K, Perch K, Chelmow D. Impaired work performance among women with symptomatic uterine fibroids. J Occup Environ Med/Am Col Occup Environ Med. 2008;50:1149–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

