Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering
- PMID: 21055807
- PMCID: PMC12497503
- DOI: 10.1016/j.biomaterials.2010.10.006
Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering
Abstract
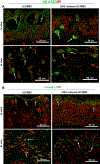
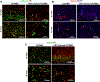
The goal of this study was to determine whether urothelial cells (UC) and smooth muscle cells (SMC) derived from the differentiation of urine-derived stem cells (USC) could be used to form engineered urethral tissue when seeded on a modified 3-D porous small intestinal submucosa (SIS) scaffold. Cells were obtained from 12 voided urine samples from 4 healthy individuals. USC were isolated, characterized and induced to differentiate into UC and SMC. Fresh SIS derived from pigs was decellularized with 5% peracetic acid (PAA). Differentiated UC and SMC derived from USC were seeded onto SIS scaffolds with highly porous microstructure in a layered co-culture fashion and cultured under dynamic conditions for one week. The seeded cells formed multiple uniform layers on the SIS and penetrated deeper into the porous matrix during dynamic culture. USC that were induced to differentiate also expressed UC markers (Uroplakin-III and AE1/AE3) or SMC markers (α-SM actin, desmin, and myosin) after implantation into athymic mice for one month, and the resulting tissues were similar to those formed when UC and SMC derived from native ureter were used. In conclusion, UC and SMC derived from USC could be maintained on 3-D porous SIS scaffold. The dynamic culture system promoted 3-D cell-matrix ingrowth and development of a multilayer mucosal structure similar to that of native urinary tract tissue. USC may serve as an alternative cell source in cell-based tissue engineering for urethral reconstruction or other urological tissue repair.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures




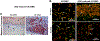
References
-
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006;367:1241–6. - PubMed
-
- Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol 2008;180:2226–33. - PubMed
-
- Bharadwaj S, Wu S, Rohozinski J, Furth M, Lan X, Atala A, et al. Multipotential differentiation of human urine-derived stem cells. J Tissue Eng Regen Med 2009;6:S293.
-
- Djordjevic ML, Majstorovic M, Stanojevic D, Bizic M, Ducic S, Kojovic V, et al. One-stage repair of severe hypospadias using combined buccal mucosa graft and longitudinal dorsal skin flap. Eur J Pediatr Surg 2008;18:427–30. - PubMed
-
- Mungadi IA, Ugboko VI. Oral mucosa grafts for urethral reconstruction. Ann Afr Med 2009;8:203–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

