Synchronized neural input shapes stimulus selectivity in a collision-detecting neuron
- PMID: 21055939
- PMCID: PMC2998055
- DOI: 10.1016/j.cub.2010.10.025
Synchronized neural input shapes stimulus selectivity in a collision-detecting neuron
Abstract
How higher-order sensory neurons generate complex selectivity from their simpler inputs is a fundamental question in neuroscience. The lobula giant movement detector (LGMD) is such a visual neuron in the locust Schistocerca americana that responds selectively to objects approaching on a collision course or their two-dimensional projections, looming stimuli [1-4]. To study how this selectivity arises, we designed an apparatus allowing us to stimulate, individually and independently, a sizable fraction of the ∼15,000 elementary visual inputs impinging retinotopically onto the LGMD's dendritic fan [5-7] (Figure 1Ai). We then recorded intracellularly in vivo throughout the visual pathway, assessing the LGMD's activity and that of all three successive presynaptic stages conveying local excitatory inputs. Our results suggest that as collision becomes increasingly imminent, the strength of these inputs increases, whereas their latency decreases. This latency decrease favors summation of inputs activated sequentially throughout the looming sequence, making the neuron maximally sensitive to collision-bound trajectories. Thus, the LGMD's selectivity arises partially from presynaptic mechanisms that synchronize a large population of inputs during a looming stimulus and subsequent detection by postsynaptic mechanisms within the neuron itself. Analogous mechanisms are likely to underlie the tuning properties of visual neurons in other species as well.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
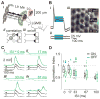

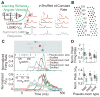
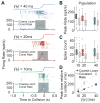
References
-
- Schlotterer GR. Response of the locust descending movement detector neuron to rapidly approaching and withdrawing visual stimuli. Can J Zool. 1977;55:1372–1376.
-
- Rind FC, Simmons PJ. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. I. Selective responses to approaching objects. J Neurophysiol. 1992;68:1654–1666. - PubMed
-
- Hatsopoulos N, Gabbiani F, Laurent G. Elementary computation of object approach by a wide-field visual neuron. Science. 1995;270:1000–1003. - PubMed
-
- Simmons PJ, Rind FC. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. II. Critical cues for detecting approaching objects. J Neurophysiol. 1992;68:1667–1682. - PubMed
-
- Strausfeld NJ, Nässel DR. Neuroarchitectures serving compound eyes of crustacea and insects. In: Autrum H, editor. Comparative physiology and evolution of vision of invertebrates, B: Invertebrate visual centers and behavior I, Handbook of sensory physiology. Vol. 7. Berlin: Springer Verlag; 1981. pp. 1–132.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

