The multiple pathways for itch and their interactions with pain
- PMID: 21056479
- PMCID: PMC2991051
- DOI: 10.1016/j.tins.2010.09.002
The multiple pathways for itch and their interactions with pain
Abstract
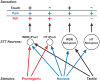
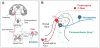
Multiple neural pathways and molecular mechanisms responsible for producing the sensation of itch have recently been identified, including histamine-independent pathways. Physiological, molecular, behavioral and brain imaging studies are converging on a description of these pathways and their close association with pain processing. Some conflicting results have arisen and the precise relationship between itch and pain remains controversial. A better understanding of the generation of itch and of the intrinsic mechanisms that inhibit itch after scratching should facilitate the search for new methods to alleviate clinical pruritus (itch). In this review we describe the current understanding of the production and inhibition of itch. A model of itch processing within the CNS is proposed.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

