Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure
- PMID: 21056571
- PMCID: PMC3019292
- DOI: 10.1016/j.yjmcc.2010.10.028
Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure
Abstract
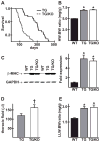
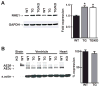
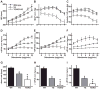
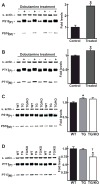
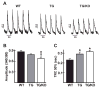
The AE3 Cl(-)/HCO(3)(-) exchanger is abundantly expressed in the sarcolemma of cardiomyocytes, where it mediates Cl(-)-uptake and HCO(3)(-)-extrusion. Inhibition of AE3-mediated Cl(-)/HCO(3)(-) exchange has been suggested to protect against cardiac hypertrophy; however, other studies indicate that AE3 might be necessary for optimal cardiac function. To test these hypotheses we crossed AE3-null mice, which appear phenotypically normal, with a hypertrophic cardiomyopathy mouse model carrying a Glu180Gly mutation in α-tropomyosin (TM180). Loss of AE3 had no effect on hypertrophy; however, survival of TM180/AE3 double mutants was sharply reduced compared with TM180 single mutants. Analysis of cardiac performance revealed impaired cardiac function in TM180 and TM180/AE3 mutants. TM180/AE3 double mutants were more severely affected and exhibited little response to β-adrenergic stimulation, a likely consequence of their more rapid progression to heart failure. Increased expression of calmodulin-dependent kinase II and protein phosphatase 1 and differences in methylation and localization of protein phosphatase 2A were observed, but were similar in single and double mutants. Phosphorylation of phospholamban on Ser16 was sharply increased in both single and double mutants relative to wild-type hearts under basal conditions, leading to reduced reserve capacity for β-adrenergic stimulation of phospholamban phosphorylation. Imaging analysis of isolated myocytes revealed reductions in amplitude and decay of Ca(2+) transients in both mutants, with greater reductions in TM180/AE3 mutants, consistent with the greater severity of their heart failure phenotype. Thus, in the TM180 cardiomyopathy model, loss of AE3 had no apparent anti-hypertrophic effect and led to more rapid decompensation and heart failure.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

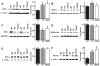

References
-
- Linn SC, Kudrycki KE, Shull GE. The predicted translation product of a cardiac AE3 mRNA contains an N terminus distinct from that of the brain AE3 Cl−/HCO3− exchanger. Cloning of a cardiac AE3 cDNA, organization of the AE3 gene, and identification of an alternative transcription initiation site. J Biol Chem. 1992;267:7927–35. - PubMed
-
- Yannoukakos D, Stuart-Tilley A, Fernandez HA, Fey P, Duyk G, Alper SL. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res. 1994;75:603–14. - PubMed
-
- Linn SC, Askew GR, Menon AG, Shull GE. Conservation of an AE3 Cl−/HCO3− exchanger cardiac-specific exon and promoter region and AE3 mRNA expression patterns in murine and human hearts. Circ Res. 1995;76:584–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

