Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease
- PMID: 21056667
- PMCID: PMC3558878
- DOI: 10.1016/j.nbd.2010.10.023
Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease
Erratum in
- Neurobiol Dis.2011 Jun;42(3):539
Abstract
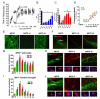
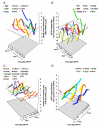
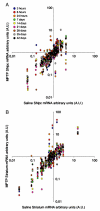
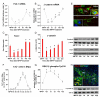
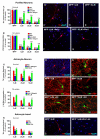
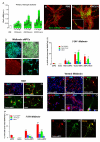
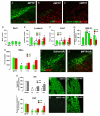
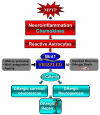
Emerging evidence points to reactive glia as a pivotal factor in Parkinson's disease (PD) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse model of basal ganglia injury, but whether astrocytes and microglia activation may exacerbate dopaminergic (DAergic) neuron demise and/or contribute to DAergic repair is presently the subject of much debate. Here, we have correlated the loss and recovery of the nigrostriatal DAergic functionality upon acute MPTP exposure with extensive gene expression analysis at the level of the ventral midbrain (VM) and striata (Str) and found a major upregulation of pro-inflammatory chemokines and wingless-type MMTV integration site1 (Wnt1), a key transcript involved in midbrain DAergic neurodevelopment. Wnt signaling components (including Frizzled-1 [Fzd-1] and β-catenin) were dynamically regulated during MPTP-induced DAergic degeneration and reactive glial activation. Activated astrocytes of the ventral midbrain were identified as candidate source of Wnt1 by in situ hybridization and real-time PCR in vitro. Blocking Wnt/Fzd signaling with Dickkopf-1 (Dkk1) counteracted astrocyte-induced neuroprotection against MPP(+) toxicity in primary mesencephalic astrocyte-neuron cultures, in vitro. Moreover, astroglial-derived factors, including Wnt1, promoted neurogenesis and DAergic neurogenesis from adult midbrain stem/neuroprogenitor cells, in vitro. Conversely, lack of Wnt1 transcription in response to MPTP in middle-aged mice and failure of DAergic neurons to recover were reversed by pharmacological activation of Wnt/β-catenin signaling, in vivo, thus suggesting MPTP-reactive astrocytes in situ and Wnt1 as candidate components of neuroprotective/neurorescue pathways in MPTP-induced nigrostriatal DAergic plasticity.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson's disease: focus on endogenous neurorestoration.Curr Aging Sci. 2013 Feb;6(1):45-55. doi: 10.2174/1874609811306010007. Curr Aging Sci. 2013. PMID: 23895521 Review.
-
Uncovering novel actors in astrocyte-neuron crosstalk in Parkinson's disease: the Wnt/β-catenin signaling cascade as the common final pathway for neuroprotection and self-repair.Eur J Neurosci. 2013 May;37(10):1550-63. doi: 10.1111/ejn.12166. Epub 2013 Mar 5. Eur J Neurosci. 2013. PMID: 23461676 Free PMC article. Review.
-
Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson's disease involves cross talk between inflammatory and Wnt/β-catenin signaling pathways: functional consequences for neuroprotection and repair.J Neurosci. 2012 Feb 8;32(6):2062-85. doi: 10.1523/JNEUROSCI.5259-11.2012. J Neurosci. 2012. PMID: 22323720 Free PMC article.
-
A Wnt1 regulated Frizzled-1/β-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: Therapeutical relevance for neuron survival and neuroprotection.Mol Neurodegener. 2011 Jul 13;6:49. doi: 10.1186/1750-1326-6-49. Mol Neurodegener. 2011. PMID: 21752258 Free PMC article.
-
Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson's Disease.Int J Mol Sci. 2018 Nov 24;19(12):3743. doi: 10.3390/ijms19123743. Int J Mol Sci. 2018. PMID: 30477246 Free PMC article. Review.
Cited by
-
Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson's disease.J Neuroinflammation. 2012 Aug 8;9:188. doi: 10.1186/1742-2094-9-188. J Neuroinflammation. 2012. PMID: 22870899 Free PMC article.
-
Conditional Haploinsufficiency of β-Catenin Aggravates Neuronal Damage in a Paraquat-Based Mouse Model of Parkinson Disease.Mol Neurobiol. 2019 Jul;56(7):5157-5166. doi: 10.1007/s12035-018-1431-z. Epub 2018 Dec 6. Mol Neurobiol. 2019. PMID: 30519817 Free PMC article.
-
"Reframing" dopamine signaling at the intersection of glial networks in the aged Parkinsonian brain as innate Nrf2/Wnt driver: Therapeutical implications.Aging Cell. 2022 Apr;21(4):e13575. doi: 10.1111/acel.13575. Epub 2022 Mar 9. Aging Cell. 2022. PMID: 35262262 Free PMC article. Review.
-
New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target?Neural Regen Res. 2020 Sep;15(9):1580-1589. doi: 10.4103/1673-5374.276320. Neural Regen Res. 2020. PMID: 32209757 Free PMC article. Review.
-
Nicotine Neurotoxicity Involves Low Wnt1 Signaling in Spinal Locomotor Networks of the Postnatal Rodent Spinal Cord.Int J Mol Sci. 2021 Sep 3;22(17):9572. doi: 10.3390/ijms22179572. Int J Mol Sci. 2021. PMID: 34502498 Free PMC article.
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;94:239–247. - PubMed
-
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006;7:207–219. - PubMed
-
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, et al. β-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. - PubMed
-
- Alvarez-Builla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2(4):2287–2293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

