Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair
- PMID: 21056998
- PMCID: PMC2993295
- DOI: 10.2353/ajpath.2010.100594
Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair
Abstract
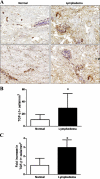
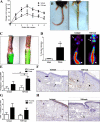
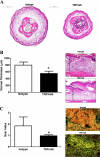
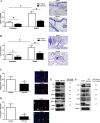
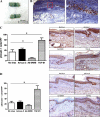
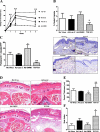
Lymphedema is a complication of cancer treatment occurring in approximately 50% of patients who undergo lymph node resection. Despite its prevalence, the etiology of this disorder remains unknown. In this study, we determined the effect of soft tissue fibrosis on lymphatic function and the role of transforming growth factor (TGF)-β1 in the regulation of this response. We determined TGF-β expression patterns in matched biopsy specimens collected from lymphedematous and normal limbs of patients with secondary lymphedema. To determine the role of TGF-β in regulating tissue fibrosis, we used a mouse model of lymphedema and inhibited TGF-β function either systemically with a monoclonal antibody or locally by using a soluble, defective TGF-β receptor. Lymphedematous tissue demonstrated a nearly threefold increase in the number of cells that stained for TGF-β1. TGF-β inhibition markedly decreased tissue fibrosis, increased lymphangiogenesis, and improved lymphatic function compared with controls. In addition, inhibition of TGF-β not only decreased TGF-β expression in lymphedematous tissues, but also diminished inflammation, migration of T-helper type 2 (Th2) cells, and expression of profibrotic Th2 cytokines. Similarly, systemic depletion of T-cells markedly decreased TGF-β expression in tail tissues. Inhibition of TGF-β function promoted lymphatic regeneration, decreased tissue fibrosis, decreased chronic inflammation and Th2 cell migration, and improved lymphatic function. The use of these strategies may represent a novel means of preventing lymphedema after lymph node resection.
Figures
References
-
- Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg. 1999;177:184–187. discussion 188. - PubMed
-
- Beaulac SM, McNair LA, Scott TE, LaMorte WW, Kavanah MT. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. 2002;137:1253–1257. - PubMed
-
- Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, Duffy SW. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–4321. - PubMed
-
- Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–1972. - PubMed
-
- Koul R, Dufan T, Russell C, Guenther W, Nugent Z, Sun X, Cooke AL. Efficacy of complete decongestive therapy and manual lymphatic drainage on treatment-related lymphedema in breast cancer. Int J Radiat Oncol Biol Phys. 2007;67:841–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

