Functional expression of γ-amino butyric acid transporter 2 in human and guinea pig airway epithelium and smooth muscle
- PMID: 21057105
- PMCID: PMC3175560
- DOI: 10.1165/rcmb.2010-0177OC
Functional expression of γ-amino butyric acid transporter 2 in human and guinea pig airway epithelium and smooth muscle
Abstract
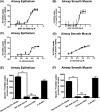
γ-Amino butyric acid (GABA) is a primary inhibitory neurotransmitter in the central nervous system, and is classically released by fusion of synaptic vesicles with the plasma membrane or by egress via GABA transporters (GATs). Recently, a GABAergic system comprised of GABA(A) and GABA(B) receptors has been identified on airway epithelial and smooth muscle cells that regulate mucus secretion and contractile tone of airway smooth muscle (ASM). In addition, the enzyme that synthesizes GABA, glutamic acid decarboxylase, has been identified in airway epithelial cells; however, the mechanism(s) by which this synthesized GABA is released from epithelial intracellular stores is unknown. We questioned whether any of the four known isoforms of GATs are functionally expressed in ASM or epithelial cells. We detected mRNA and protein expression of GAT2 and -4, and isoforms of glutamic acid decarboxylase in native and cultured human ASM and epithelial cells. In contrast, mRNA encoding vesicular GAT (VGAT), the neuronal GABA transporter, was not detected. Functional inhibition of (3)H-GABA uptake was demonstrated using GAT2 and GAT4/betaine-GABA transporter 1 (BGT1) inhibitors in both human ASM and epithelial cells. These results demonstrate that two isoforms of GATs, but not VGAT, are expressed in both airway epithelial and smooth muscle cells. They also provide a mechanism by which locally synthesized GABA can be released from these cells into the airway to activate GABA(A) channels and GABA(B) receptors, with subsequent autocrine and/or paracrine signaling effects on airway epithelium and ASM.
Figures
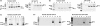


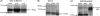


Similar articles
-
Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone.Am J Physiol Lung Cell Mol Physiol. 2013 Feb 1;304(3):L191-7. doi: 10.1152/ajplung.00274.2012. Epub 2012 Nov 30. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23204068 Free PMC article.
-
Functional expression of GABAB receptors in airway epithelium.Am J Respir Cell Mol Biol. 2008 Sep;39(3):296-304. doi: 10.1165/rcmb.2007-0414OC. Epub 2008 Apr 10. Am J Respir Cell Mol Biol. 2008. PMID: 18403780 Free PMC article.
-
GABAA receptors are expressed and facilitate relaxation in airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2008 Jun;294(6):L1206-16. doi: 10.1152/ajplung.00287.2007. Epub 2008 Apr 11. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 18408071 Free PMC article.
-
Structural and molecular aspects of betaine-GABA transporter 1 (BGT1) and its relation to brain function.Neuropharmacology. 2019 Dec 15;161:107644. doi: 10.1016/j.neuropharm.2019.05.021. Epub 2019 May 18. Neuropharmacology. 2019. PMID: 31108110 Review.
-
Development of Non-GAT1-Selective Inhibitors: Challenges and Achievements.Adv Neurobiol. 2017;16:315-332. doi: 10.1007/978-3-319-55769-4_16. Adv Neurobiol. 2017. PMID: 28828618 Review.
Cited by
-
Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease.Am J Physiol Lung Cell Mol Physiol. 2016 Dec 1;311(6):L1113-L1140. doi: 10.1152/ajplung.00370.2016. Epub 2016 Oct 14. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27742732 Free PMC article. Review.
-
Airway smooth muscle in airway reactivity and remodeling: what have we learned?Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(12):L912-33. doi: 10.1152/ajplung.00259.2013. Epub 2013 Oct 18. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24142517 Free PMC article. Review.
-
Anesthetic effects on γ-aminobutyric acid A receptors: not just on your nerves.Anesthesiology. 2013 May;118(5):1013-5. doi: 10.1097/ALN.0b013e31828e1865. Anesthesiology. 2013. PMID: 23612125 Free PMC article. No abstract available.
-
Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone.Am J Physiol Lung Cell Mol Physiol. 2013 Feb 1;304(3):L191-7. doi: 10.1152/ajplung.00274.2012. Epub 2012 Nov 30. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23204068 Free PMC article.
-
Airway Epithelial Cell Release of GABA is Regulated by Protein Kinase A.Lung. 2016 Jun;194(3):401-8. doi: 10.1007/s00408-016-9867-2. Epub 2016 Mar 17. Lung. 2016. PMID: 26989055 Free PMC article.
References
-
- Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol 2005;94:2073–2085. - PubMed
-
- Bragina L, Marchionni I, Omrani A, Cozzi A, Pellegrini-Giampietro DE, Cherubini E, Conti F. GAT-1 regulates both tonic and phasic GABA(A) receptor-mediated inhibition in the cerebral cortex. J Neurochem 2008;105:1781–1793. - PubMed
-
- Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J Membr Biol 2006;213:89–100. - PubMed
-
- Kempson SA, Parikh V, Xi L, Chu S, Montrose MH. Subcellular redistribution of the renal betaine transporter during hypertonic stress. Am J Physiol Cell Physiol 2003;285:C1091–C1100. - PubMed
-
- Olsen M, Sarup A, Larsson OM, Schousboe A. Effect of hyperosmotic conditions on the expression of the betaine–GABA–transporter (BGT-1) in cultured mouse astrocytes. Neurochem Res 2005;30:855–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

