Phosphorylation-independent dual-site binding of the FHA domain of KIF13 mediates phosphoinositide transport via centaurin alpha1
- PMID: 21057110
- PMCID: PMC2996657
- DOI: 10.1073/pnas.1009008107
Phosphorylation-independent dual-site binding of the FHA domain of KIF13 mediates phosphoinositide transport via centaurin alpha1
Abstract
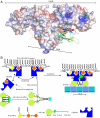
Phosphatidylinositol 3,4,5-triphosphate (PIP3) plays a key role in neuronal polarization and axon formation. PIP3-containing vesicles are transported to axon tips by the kinesin KIF13B via an adaptor protein, centaurin α1 (CENTA1). KIF13B interacts with CENTA1 through its forkhead-associated (FHA) domain. We solved the crystal structures of CENTA1 in ligand-free, KIF13B-FHA domain-bound, and PIP3 head group (IP4)-bound conformations, and the CENTA1/KIF13B-FHA/IP4 ternary complex. The first pleckstrin homology (PH) domain of CENTA1 specifically binds to PIP3, while the second binds to both PIP3 and phosphatidylinositol 3,4-biphosphate (PI(3,4)P(2)). The FHA domain of KIF13B interacts with the PH1 domain of one CENTA1 molecule and the ArfGAP domain of a second CENTA1 molecule in a threonine phosphorylation-independent fashion. We propose that full-length KIF13B and CENTA1 form heterotetramers that can bind four phosphoinositide molecules in the vesicle and transport it along the microtubule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Centaurin-alpha1 interacts directly with kinesin motor protein KIF13B.J Cell Sci. 2005 Jun 1;118(Pt 11):2471-84. doi: 10.1242/jcs.02369. J Cell Sci. 2005. PMID: 15923660
-
In vitro reconstitution reveals phosphoinositides as cargo-release factors and activators of the ARF6 GAP ADAP1.Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2010054118. doi: 10.1073/pnas.2010054118. Epub 2020 Dec 18. Proc Natl Acad Sci U S A. 2021. PMID: 33443153 Free PMC article.
-
Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity.J Cell Biol. 2006 Jul 31;174(3):425-36. doi: 10.1083/jcb.200604031. Epub 2006 Jul 24. J Cell Biol. 2006. PMID: 16864656 Free PMC article.
-
Centaurin-alpha1 and KIF13B kinesin motor protein interaction in ARF6 signalling.Biochem Soc Trans. 2005 Dec;33(Pt 6):1279-81. doi: 10.1042/BST0331279. Biochem Soc Trans. 2005. PMID: 16246098 Review.
-
Functions of the neuron-specific protein ADAP1 (centaurin-α1) in neuronal differentiation and neurodegenerative diseases, with an overview of structural and biochemical properties of ADAP1.Biol Chem. 2014 Nov 1;395(11):1321-40. doi: 10.1515/hsz-2014-0107. Biol Chem. 2014. PMID: 24854535 Review.
Cited by
-
Intracellular Cargo Transport by Kinesin-3 Motors.Biochemistry (Mosc). 2017 Jul;82(7):803-815. doi: 10.1134/S0006297917070057. Biochemistry (Mosc). 2017. PMID: 28918744 Free PMC article. Review.
-
Cyclin-dependent kinase 5 controls TRPV1 membrane trafficking and the heat sensitivity of nociceptors through KIF13B.J Neurosci. 2012 Oct 17;32(42):14709-21. doi: 10.1523/JNEUROSCI.1634-12.2012. J Neurosci. 2012. PMID: 23077056 Free PMC article.
-
Significant reduction in errors associated with nonbonded contacts in protein crystal structures: automated all-atom refinement with PrimeX.Acta Crystallogr D Biol Crystallogr. 2012 Aug;68(Pt 8):935-52. doi: 10.1107/S0907444912017453. Epub 2012 Jul 17. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 22868759 Free PMC article.
-
Functional Role of AGAP2/PIKE-A in Fcγ Receptor-Mediated Phagocytosis.Cells. 2022 Dec 24;12(1):72. doi: 10.3390/cells12010072. Cells. 2022. PMID: 36611866 Free PMC article.
-
Afadin couples RAS GTPases to the polarity rheostat Scribble.Nat Commun. 2022 Aug 5;13(1):4562. doi: 10.1038/s41467-022-32335-8. Nat Commun. 2022. PMID: 35931706 Free PMC article.
References
-
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. - PubMed
-
- Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. - PubMed
-
- Menager C, Arimura N, Fukata Y, Kaibuchi K. PIP3 is involved in neuronal polarization and axon formation. J Neurochem. 2004;89:109–118. - PubMed
-
- Mahajan A, et al. Structure and function of the phosphothreonine-specific FHA domain. Science Signaling. 2008;1:re12. http://www.ncbi.nlm.nih.gov/pubmed/19109241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

