Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency
- PMID: 21057262
- PMCID: PMC3103888
- DOI: 10.1097/MD.0b013e3181fd8ec3
Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency
Abstract
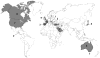
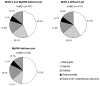
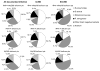
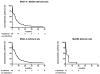
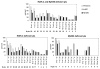
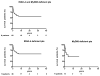
Autosomal recessive interleukin-1 receptor-associated kinase (IRAK)-4 and myeloid differentiation factor (MyD)88 deficiencies impair Toll-like receptor (TLR)- and interleukin-1 receptor-mediated immunity. We documented the clinical features and outcome of 48 patients with IRAK-4 deficiency and 12 patients with MyD88 deficiency, from 37 kindreds in 15 countries.The clinical features of IRAK-4 and MyD88 deficiency were indistinguishable. There were no severe viral, parasitic, and fungal diseases, and the range of bacterial infections was narrow. Noninvasive bacterial infections occurred in 52 patients, with a high incidence of infections of the upper respiratory tract and the skin, mostly caused by Pseudomonas aeruginosa and Staphylococcus aureus, respectively. The leading threat was invasive pneumococcal disease, documented in 41 patients (68%) and causing 72 documented invasive infections (52.2%). P. aeruginosa and Staph. aureus documented invasive infections also occurred (16.7% and 16%, respectively, in 13 and 13 patients, respectively). Systemic signs of inflammation were usually weak or delayed. The first invasive infection occurred before the age of 2 years in 53 (88.3%) and in the neonatal period in 19 (32.7%) patients. Multiple or recurrent invasive infections were observed in most survivors (n = 36/50, 72%).Clinical outcome was poor, with 24 deaths, in 10 cases during the first invasive episode and in 16 cases of invasive pneumococcal disease. However, no death and invasive infectious disease were reported in patients after the age of 8 years and 14 years, respectively. Antibiotic prophylaxis (n = 34), antipneumococcal vaccination (n = 31), and/or IgG infusion (n = 19), when instituted, had a beneficial impact on patients until the teenage years, with no seemingly detectable impact thereafter.IRAK-4 and MyD88 deficiencies predispose patients to recurrent life-threatening bacterial diseases, such as invasive pneumococcal disease in particular, in infancy and early childhood, with weak signs of inflammation. Patients and families should be informed of the risk of developing life-threatening infections; empiric antibacterial treatment and immediate medical consultation are strongly recommended in cases of suspected infection or moderate fever. Prophylactic measures in childhood are beneficial, until spontaneous improvement occurs in adolescence.
Figures
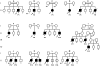
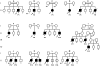
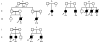

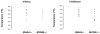
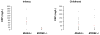

References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. - PubMed
-
- Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JL, Bont L. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–237. - PMC - PubMed
-
- Borgers H, Moens L, Picard C, Jeurissen A, Raes M, Sauer K, Proesmans M, De Boeck K, Casanova JL, Meyts I, Bossuyt X. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol. 2010;134:198–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

