A role for IL-27p28 as an antagonist of gp130-mediated signaling
- PMID: 21057510
- PMCID: PMC3059498
- DOI: 10.1038/ni.1957
A role for IL-27p28 as an antagonist of gp130-mediated signaling
Abstract
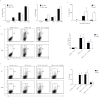
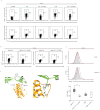
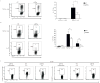
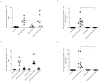
The heterodimeric cytokine interleukin 27 (IL-27) signals through the IL-27Rα subunit of its receptor, combined with gp130, a common receptor chain used by several cytokines, including IL-6. Notably, the IL-27 subunits p28 (IL-27p28) and EBI3 are not always expressed together, which suggests that they may have unique functions. Here we show that IL-27p28, independently of EBI3, antagonized cytokine signaling through gp130 and IL-6-mediated production of IL-17 and IL-10. Similarly, the ability to generate antibody responses was dependent on the activity of gp130-signaling cytokines. Mice transgenic for expression of IL-27p28 showed a substantial defect in the formation of germinal centers and antibody production. Thus, IL-27p28, as a natural antagonist of gp130-mediated signaling, may be useful as a therapeutic for managing inflammation mediated by cytokines that signal through gp130.
Figures


References
-
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. - PubMed
-
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. - PubMed
-
- Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. 2005;25:241–253. - PubMed
-
- Ding C, Cicuttini F, Li J, Jones G. Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opin Investig Drugs. 2009;18:1457–1466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

