Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria
- PMID: 21057511
- PMCID: PMC3058225
- DOI: 10.1038/ni.1960
Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria
Abstract
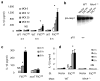
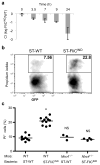
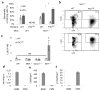
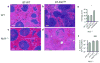
Macrophages mediate crucial innate immune responses via caspase-1-dependent processing and secretion of interleukin 1β (IL-1β) and IL-18. Although infection with wild-type Salmonella typhimurium is lethal to mice, we show here that a strain that persistently expresses flagellin was cleared by the cytosolic flagellin-detection pathway through the activation of caspase-1 by the NLRC4 inflammasome; however, this clearance was independent of IL-1β and IL-18. Instead, caspase-1-induced pyroptotic cell death released bacteria from macrophages and exposed the bacteria to uptake and killing by reactive oxygen species in neutrophils. Similarly, activation of caspase-1 cleared unmanipulated Legionella pneumophila and Burkholderia thailandensis by cytokine-independent mechanisms. This demonstrates that activation of caspase-1 clears intracellular bacteria in vivo independently of IL-1β and IL-18 and establishes pyroptosis as an efficient mechanism of bacterial clearance by the innate immune system.
Figures

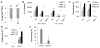
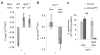
References
-
- Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. - PubMed
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. - PubMed
-
- Kozak W, et al. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am J Physiol. 1995;269:R969–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

