The mechanism of sperm-egg interaction and the involvement of IZUMO1 in fusion
- PMID: 21057513
- PMCID: PMC3739395
- DOI: 10.1038/aja.2010.70
The mechanism of sperm-egg interaction and the involvement of IZUMO1 in fusion
Abstract
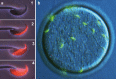
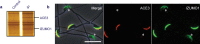
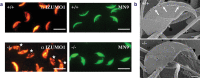
An average human ejaculate contains over 100 million sperm, but only a few succeed in accomplishing the journey to an egg by migration through the female reproductive tract. Among these few sperm, only one participates in fertilization. There might be an ingenious molecular mechanism to ensure that the very best sperm fertilize an egg. However, recent gene disruption experiments in mice have revealed that many factors previously described as important for fertilization are largely dispensable. One could argue that the fertilization mechanism is made robust against gene disruptions. However, this is not likely, as there are already six different gene-disrupted mouse lines (Calmegin, Adam1a, Adam2, Adam3, Ace and Pgap1), all of which result in male sterility. The sperm from these animals are known to have defective zona-binding ability and at the same time lose oviduct-migrating ability. Concerning sperm-zona binding, the widely accepted involvement of sugar moiety on zona pellucida 3 (ZP3) is indicated to be dispensable by gene disruption experiments. Thus, the landscape of the mechanism of fertilization is revolving considerably. In the sperm-egg fusion process, CD9 on egg and IZUMO1 on sperm have emerged as essential factors. This review focuses on the mechanism of fertilization elucidated by gene-manipulated animals.
Figures


Similar articles
-
Mechanisms of fertilization--a view from the study of gene-manipulated mice.J Androl. 2011 May-Jun;32(3):218-25. doi: 10.2164/jandrol.110.010900. Epub 2010 Oct 21. J Androl. 2011. PMID: 20966425 Review.
-
Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion.Development. 2013 Aug;140(15):3221-9. doi: 10.1242/dev.094854. Epub 2013 Jul 3. Development. 2013. PMID: 23824580
-
Cell adhesion and fertilization: steps in oocyte transport, sperm-zona pellucida interactions, and sperm-egg fusion.Biol Reprod. 2003 Jan;68(1):1-9. doi: 10.1095/biolreprod.102.007856. Biol Reprod. 2003. PMID: 12493688 Review.
-
Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding.Nature. 1992 Jun 18;357(6379):589-93. doi: 10.1038/357589a0. Nature. 1992. PMID: 1608469
-
Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (Clgn)-deficient mice.Biol Reprod. 2006 Nov;75(5):760-6. doi: 10.1095/biolreprod.106.052977. Epub 2006 Jul 26. Biol Reprod. 2006. PMID: 16870943
Cited by
-
Decoding the Genes Orchestrating Egg and Sperm Fusion Reactions and Their Roles in Fertility.Biomedicines. 2024 Dec 15;12(12):2850. doi: 10.3390/biomedicines12122850. Biomedicines. 2024. PMID: 39767756 Free PMC article. Review.
-
The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review.Int J Mol Sci. 2022 Apr 1;23(7):3929. doi: 10.3390/ijms23073929. Int J Mol Sci. 2022. PMID: 35409288 Free PMC article.
-
Identification of bovine sperm acrosomal proteins that interact with a 32-kDa acrosomal matrix protein.Mol Cell Biochem. 2016 Mar;414(1-2):153-69. doi: 10.1007/s11010-016-2668-3. Epub 2016 Feb 20. Mol Cell Biochem. 2016. PMID: 26897631 Free PMC article.
-
The vertebrate- and testis- specific transmembrane protein C11ORF94 plays a critical role in sperm-oocyte membrane binding.Mol Biomed. 2022 Sep 2;3(1):27. doi: 10.1186/s43556-022-00092-1. Mol Biomed. 2022. PMID: 36050562 Free PMC article.
-
CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization.Proc Natl Acad Sci U S A. 2011 Jul 5;108(27):10946-51. doi: 10.1073/pnas.1017400108. Epub 2011 Jun 20. Proc Natl Acad Sci U S A. 2011. PMID: 21690351 Free PMC article.
References
-
- Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem. 1994;269:31845–9. - PubMed
-
- Lu Q, Shur BD. Sperm from beta 1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development. 1997;124:4121–31. - PubMed
-
- He ZY, Brakebusch C, Fassler R, Kreidberg JA, Primakoff P, et al. None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm–egg binding and fusion. Dev Biol. 2003;254:226–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

