Mouse models in male fertility research
- PMID: 21057516
- PMCID: PMC3739380
- DOI: 10.1038/aja.2010.101
Mouse models in male fertility research
Abstract
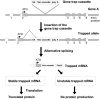
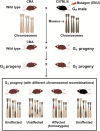
Limited knowledge of the genetic causes of male infertility has resulted in few treatment and targeted therapeutic options. Although the ideal approach to identify infertility causing mutations is to conduct studies in the human population, this approach has progressed slowly due to the limitations described herein. Given the complexity of male fertility, the entire process cannot be modeled in vitro. As such, animal models, in particular mouse models, provide a valuable alternative for gene identification and experimentation. Since the introduction of molecular biology and recent advances in animal model production, there has been a substantial acceleration in the identification and characterization of genes associated with many diseases, including infertility. Three major types of mouse models are commonly used in biomedical research, including knockout/knockin/gene-trapped, transgenic and chemical-induced point mutant mice. Using these mouse models, over 400 genes essential for male fertility have been revealed. It has, however, been estimated that thousands of genes are involved in the regulation of the complex process of male fertility, as many such genes remain to be characterized. The current review is by no means a comprehensive list of these mouse models, rather it contains examples of how mouse models have advanced our knowledge of post-natal germ cell development and male fertility regulation.
Figures



References
-
- Leifke E, Nieschlag E. Male infertility treatment in the light of evidence-based medicine. Andrologia. 1996;28 Suppl 1:23–30. - PubMed
-
- de Kretser DM. Male infertility. Lancet. 1997;349:787–90. - PubMed
-
- de Kretser DM, Baker HW. Infertility in men: recent advances and continuing controversies. J Clin Endocrinol Metab. 1999;84:3443–50. - PubMed
-
- O'Flynn O'Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010;93:1–12. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

