p85α mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response
- PMID: 21057544
- PMCID: PMC3761396
- DOI: 10.1038/onc.2010.506
p85α mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response
Abstract
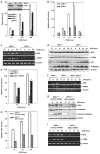
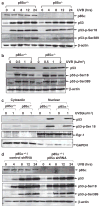
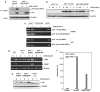
Inducible acetylation of p53 at lysine residues has a great impact on regulating the transactivation of this protein, which is associated with cell growth arrest and/or apoptosis under various stress conditions. However, the factor(s) for regulating p53 acetylation remains largely unknown. In the current study, we have shown that p85α, the regulatory subunit of phosphatidylinositol-3-kinase, has a critical role in mediating p53 acetylation and promoter-specific transactivation in the ultraviolet B (UVB) response. Depletion of p85α in mouse embryonic fibroblasts significantly impairs UVB-induced apoptosis, as well as p53 transactivation and acetylation at Lys370 (Lys373 of human p53); however, the accumulation, nuclear translocation and phosphorylation of p53 are not affected. Interestingly, p85α binds to p300, promotes the p300-p53 interaction and the subsequent recruitment of the p53/p300 complex to the promoter region of the specific p53 target gene in response to UVB irradiation. Moreover, ablation of p53 acetylation at Lys370 by site-directed mutagenesis dramatically suppresses UVB-induced expression of the specific p53-responsive gene as well as cell apoptosis. Therefore, we conclude that p85α is a novel regulator of p53-mediated response under certain stress conditions, and targeting the p85α-dependent p53 pathway may be promising for cancer therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
p85α mediates NFAT3-dependent VEGF induction in the cellular UVB response.J Cell Sci. 2013 Mar 15;126(Pt 6):1317-22. doi: 10.1242/jcs.115550. Epub 2013 Feb 6. J Cell Sci. 2013. PMID: 23390317
-
Skp2 suppresses p53-dependent apoptosis by inhibiting p300.Mol Cell. 2008 Feb 1;29(2):217-31. doi: 10.1016/j.molcel.2007.11.036. Mol Cell. 2008. PMID: 18243116
-
IKKα mediates UVB-induced cell apoptosis by regulating p53 pathway activation.Ecotoxicol Environ Saf. 2021 Dec 20;227:112892. doi: 10.1016/j.ecoenv.2021.112892. Epub 2021 Oct 11. Ecotoxicol Environ Saf. 2021. PMID: 34649141
-
The transcriptional coactivator and acetyltransferase p300 in fibroblast biology and fibrosis.J Cell Physiol. 2007 Dec;213(3):663-71. doi: 10.1002/jcp.21162. J Cell Physiol. 2007. PMID: 17559085 Review.
-
p300/CBP/p53 interaction and regulation of the p53 response.Eur J Biochem. 2001 May;268(10):2773-8. doi: 10.1046/j.1432-1327.2001.02226.x. Eur J Biochem. 2001. PMID: 11358491 Review.
Cited by
-
The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP.J Biol Chem. 2012 Oct 12;287(42):35234-35243. doi: 10.1074/jbc.M112.389494. Epub 2012 Aug 15. J Biol Chem. 2012. PMID: 22896709 Free PMC article.
-
High Frequency of PIK3R1 Alterations in Ovarian Cancers: Clinicopathological and Molecular Associations.Cancers (Basel). 2025 Jan 15;17(2):269. doi: 10.3390/cancers17020269. Cancers (Basel). 2025. PMID: 39858051 Free PMC article.
-
Identification of druggable targets for radiation mitigation using a small interfering RNA screening assay.Radiat Res. 2012 Sep;178(3):150-9. doi: 10.1667/rr2810.1. Epub 2012 Jul 2. Radiat Res. 2012. PMID: 22747550 Free PMC article.
-
Ribosomal protein S7 regulates arsenite-induced GADD45α expression by attenuating MDM2-mediated GADD45α ubiquitination and degradation.Nucleic Acids Res. 2013 May 1;41(10):5210-22. doi: 10.1093/nar/gkt223. Epub 2013 Apr 5. Nucleic Acids Res. 2013. PMID: 23563151 Free PMC article.
-
Potential molecular characteristics in situ in response to repetitive UVB irradiation.Diagn Pathol. 2016 Nov 10;11(1):129. doi: 10.1186/s13000-016-0579-y. Diagn Pathol. 2016. PMID: 27829444 Free PMC article.
References
-
- An W, Kim J, Roeder RG. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell. 2004;117:735–748. - PubMed
-
- Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. - PubMed
-
- Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol. 2003;15:164–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

