How Do Atrial-Selective Drugs Differ From Antiarrhythmic Drugs Currently Used in the Treatment of Atrial Fibrillation?
- PMID: 21057583
- PMCID: PMC2970929
- DOI: 10.4022/jafib.v1i1.400
How Do Atrial-Selective Drugs Differ From Antiarrhythmic Drugs Currently Used in the Treatment of Atrial Fibrillation?
Abstract
Current pharmacologic strategies for the management of Atrial fibrillation (AF) include use of 1) sodium channel blockers, which are contraindicated in patients with coronary artery or structural heart disease because of their potent effect to slow conduction in the ventricles, 2) potassium channel blockers, which predispose to acquired long QT and Torsade de Pointes arrhythmias because of their potent effect to prolong ventricular repolarization, and 3) mixed ion channel blockers such as amiodarone, which are associated with multi-organ toxicity. Accordingly, recent studies have focused on agents that selectively affect the atria but not the ventricles. Several Atrial-selective approaches have been proposed for the management of AF, including inhibition of the Atrial-specific ultra rapid delayed rectified potassium current (IKur), acetylcholine-regulated inward rectifying potassium current (IK-ACh), or connexin-40 (Cx40). All three are largely exclusive to atria. Recent studies have proposed that an Atrial-selective depression of sodium channel-dependent parameters with agents such as ranolazine may be an alternative approach capable of effectively suppressing AF without increasing susceptibility to ventricular arrhythmias. Clinical evidence for Cx40 modulation or IK-ACh inhibition are lacking at this time. The available data suggest that Atrial-selective approaches involving a combination of INa, IKur, IKr, and, perhaps, Ito block may be more effective in the management of AF than pure IKur or INa block. The anti-AF efficacy of the Atrial-selective/predominant agents appears to be similar to that of conventionally used anti-AF agents, with the major apparent difference being that the latter are associated with ventricular arrhythmogenesis and extra cardiac toxicity.
Figures
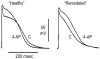
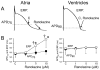

References
-
- Wang ZG, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes: Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. - PubMed
-
- Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov. 2006;5:1034–1049. - PubMed
-
- Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, Christ T, Schuler S, Ravens U. Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. - PubMed
-
- Ehrlich JR, Biliczki P, Hohnloser SH, Nattel S. Atrial-selective approaches for the treatment of atrial fibrillation. J Am Coll Cardiol. 2008;51:787–792. - PubMed
-
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
