Regioselective Diels-Alder cycloadditions and other reactions of 4,5-, 5,6-, and 6,7-indole arynes
- PMID: 21057588
- PMCID: PMC2971556
- DOI: 10.1016/j.tetlet.2008.10.086
Regioselective Diels-Alder cycloadditions and other reactions of 4,5-, 5,6-, and 6,7-indole arynes
Abstract
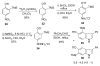
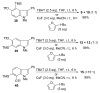
The regioselectivity of Diels-Alder cycloadditions of indole arynes (indolynes) at all three benzenoid positions was examined. Cycloadditions with the 4,5-and 5,6-indolynes, derived via metal-halogen exchange from the corresponding o-dibromo indoles, showed essentially no selectivity with 2-t-butylfuran. In contrast, the 6,7-indolyne displayed virtually complete preference for the more sterically congested cycloadduct. This same cycloadduct undergoes a facile acid-catalyzed rearrangement to afford the annulated enone, or alternatively, undergoes hydrolysis and oxidation in the presence of air to give the indolobenzoquinone. The 5,6-difluoroindoles show anomolous behavior and give either 5-fluoro-6,7-indolynes with n-BuLi in ether, or 5,6-indolynes with n-BuLi in toluene. We have also demonstrated that benzenoid indolynes can be easily and conveniently generated by the fluoride-induced decomposition of o-trimethylsilyl triflates.
Figures


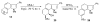
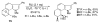
Similar articles
-
Indole-derived arynes and their Diels-Alder reactivity with furans.Org Lett. 2007 Oct 11;9(21):4135-7. doi: 10.1021/ol701595n. Epub 2007 Sep 19. Org Lett. 2007. PMID: 17880092
-
Regioselectivity of Diels-Alder Reactions Between 6,7-Dehydrobenzofuran and 2-Substituted Furans.Tetrahedron Lett. 2012 Aug 1;53(31):4022-4025. doi: 10.1016/j.tetlet.2012.05.118. Tetrahedron Lett. 2012. PMID: 25278635 Free PMC article.
-
Experimental and theoretical investigations into the unusual regioselectivity of 4,5-, 5,6-, and 6,7-indole aryne cycloadditions.Org Lett. 2010 Jan 1;12(1):96-9. doi: 10.1021/ol902415s. Org Lett. 2010. PMID: 19961152 Free PMC article.
-
[The chemistry of pericyclic reactions and their application to syntheses of heterocyclic compounds].Yakugaku Zasshi. 2003 Sep;123(9):717-59. doi: 10.1248/yakushi.123.717. Yakugaku Zasshi. 2003. PMID: 14513766 Review. Japanese.
-
Pyridynes and indolynes as building blocks for functionalized heterocycles and natural products.Chem Commun (Camb). 2015 Jan 4;51(1):34-45. doi: 10.1039/c4cc06445c. Chem Commun (Camb). 2015. PMID: 25226878 Review.
Cited by
-
Asymmetric hydrovinylation of vinylindoles. A facile route to cyclopenta[g]indole natural products (+)-cis-trikentrin A and (+)-cis-trikentrin B.J Am Chem Soc. 2012 Mar 28;134(12):5496-9. doi: 10.1021/ja3004733. Epub 2012 Mar 13. J Am Chem Soc. 2012. PMID: 22394308 Free PMC article.
-
Total Synthesis of (±)-Cis-Trikentrin B via Intermolecular 6,7-Indole Aryne Cycloaddition and Stille Cross-Coupling.Tetrahedron Lett. 2013 Feb 20;54(8):913-917. doi: 10.1016/j.tetlet.2012.11.125. Tetrahedron Lett. 2013. PMID: 25278636 Free PMC article.
-
Indolyne experimental and computational studies: synthetic applications and origins of selectivities of nucleophilic additions.J Am Chem Soc. 2010 Dec 22;132(50):17933-44. doi: 10.1021/ja1086485. Epub 2010 Nov 29. J Am Chem Soc. 2010. PMID: 21114321 Free PMC article.
-
Total syntheses of indolactam alkaloids (-)-indolactam V, (-)-pendolmycin, (-)-lyngbyatoxin A, and (-)-teleocidin A-2.Chem Sci. 2014 Jun 1;5(6):2184-2190. doi: 10.1039/C4SC00256C. Chem Sci. 2014. PMID: 24839542 Free PMC article.
-
Oxa- and Azabenzonorbornadienes as Electrophilic Partners under Photoredox/Nickel Dual Catalysis.ACS Catal. 2019 Sep 6;9(9):8835-8842. doi: 10.1021/acscatal.9b02458. Epub 2019 Aug 28. ACS Catal. 2019. PMID: 34055458 Free PMC article.
References
-
-
Roberts JD, Simmons HE, Jr, Carlsmith LA, Vaughn CW. J Am Chem Soc. 1953;75:3290–3291.Roberts JD, Semenow DA, Simmons HE, Jr, Carlsmith LA. J Am Chem Soc. 1956;78:601–611.For a review of arynes in general, see: Hoffmann RW. Dehydrobenzene and Cycloalkynes. Academic; New York: 1967.
-
-
- Bunnett JF, Brotherton TK. J Org Chem. 1958;23:904–906.
- Huisgen R, Zirngibl L. Chem Ber. 1958;91:1438–1452.
-
- Kauffmann T, Boettcher FP. Angew Chem. 1961;73:65–66.
- Martens RJ, den Hertog HJ. Tetrahedron Lett. 1962:643–645.
-
- Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org Lett. 2007;9:4135–4137. - PubMed
-
-
For attempts to generate the elusive 2,3-indolyne, see: Conway SC, Gribble GW. Heterocycles. 1992;34:2095–2108.Gribble GW, Conway SC. Synth Commun. 1992;22:2129–2141.
-
Grants and funding
LinkOut - more resources
Full Text Sources
