Auditory cortex electrical stimulation suppresses tinnitus in rats
- PMID: 21057850
- PMCID: PMC3046330
- DOI: 10.1007/s10162-010-0246-z
Auditory cortex electrical stimulation suppresses tinnitus in rats
Abstract
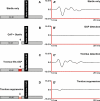
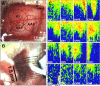
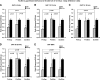
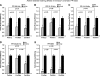
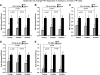
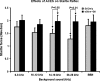
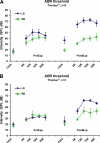
Recent clinical studies have demonstrated that auditory cortex electrical stimulation (ACES) has yielded promising results in the suppression of patients' tinnitus. However, the large variability in the efficacy of ACES-induced suppression across individuals has hindered its development into a reliable therapy. Due to ethical reasons, many issues cannot be comprehensively addressed in patients. In order to search for effective stimulation targets and identify optimal stimulation strategies, we have developed the first rat model to test for the suppression of behavioral evidence of tone-induced tinnitus through ACES. Our behavioral results demonstrated that electrical stimulation of all channels (frequency bands) in the auditory cortex significantly suppressed behavioral evidence of tinnitus and enhanced hearing detection at the central level. Such suppression of tinnitus and enhancement of hearing detection were respectively demonstrated by a reversal of tone exposure compromised gap detection at 10-12, 14-16, and 26-28 kHz and compromised prepulse inhibition at 10-12 and 26-28 kHz. On the contrary, ACES did not induce behavioral changes in animals that did not manifest any behavioral evidence of tinnitus and compromised hearing detection following the same tone exposure. The results point out that tinnitus may be more related to compromised central auditory processing than hearing loss at the peripheral level. The ACES-induced suppression of behavioral evidence of tinnitus may involve restoration of abnormal central auditory processing.
Figures

Similar articles
-
Primary and secondary auditory cortex stimulation for intractable tinnitus.ORL J Otorhinolaryngol Relat Spec. 2006;68(1):48-54; discussion 54-5. doi: 10.1159/000090491. Epub 2006 Mar 3. ORL J Otorhinolaryngol Relat Spec. 2006. PMID: 16514263
-
Hearing suppression induced by electrical stimulation of human auditory cortex.Brain Res. 2006 Nov 6;1118(1):75-83. doi: 10.1016/j.brainres.2006.08.013. Epub 2006 Sep 18. Brain Res. 2006. PMID: 16979144 Free PMC article.
-
Electrical stimulation of auditory and somatosensory cortices for treatment of tinnitus and pain.Prog Brain Res. 2007;166:377-88. doi: 10.1016/S0079-6123(07)66036-1. Prog Brain Res. 2007. PMID: 17956802 Clinical Trial.
-
Auditory cortex stimulation to suppress tinnitus: mechanisms and strategies.Hear Res. 2013 Jan;295:38-57. doi: 10.1016/j.heares.2012.05.007. Epub 2012 Jun 6. Hear Res. 2013. PMID: 22683861 Review.
-
Auditory cortex stimulation for tinnitus.Acta Neurochir Suppl. 2007;97(Pt 2):451-62. doi: 10.1007/978-3-211-33081-4_52. Acta Neurochir Suppl. 2007. PMID: 17691335 Review.
Cited by
-
Animal Models of Tinnitus Treatment: Cochlear and Brain Stimulation.Curr Top Behav Neurosci. 2021;51:83-129. doi: 10.1007/7854_2021_227. Curr Top Behav Neurosci. 2021. PMID: 34282563
-
Animal models of subjective tinnitus.Neural Plast. 2014;2014:741452. doi: 10.1155/2014/741452. Epub 2014 Apr 16. Neural Plast. 2014. PMID: 24829805 Free PMC article. Review.
-
GLAST Deficiency in Mice Exacerbates Gap Detection Deficits in a Model of Salicylate-Induced Tinnitus.Front Behav Neurosci. 2016 Aug 17;10:158. doi: 10.3389/fnbeh.2016.00158. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27582696 Free PMC article.
-
Strain Comparison in Rats Differentiates Strain-Specific from More General Correlates of Noise-Induced Hearing Loss and Tinnitus.J Assoc Res Otolaryngol. 2022 Feb;23(1):59-73. doi: 10.1007/s10162-021-00822-2. Epub 2021 Nov 18. J Assoc Res Otolaryngol. 2022. PMID: 34796410 Free PMC article.
-
Effects of furosemide on cochlear neural activity, central hyperactivity and behavioural tinnitus after cochlear trauma in guinea pig.PLoS One. 2014 May 16;9(5):e97948. doi: 10.1371/journal.pone.0097948. eCollection 2014. PLoS One. 2014. PMID: 24835470 Free PMC article.
References
-
- Ashton H, Reid K, Marsh R, Johnson I, Alter K, Griffiths T. High frequency localised “hot spots” in temporal lobes of patients with intractable tinnitus: a quantitative electroencephalographic (QEEG) study. Neurosci Lett. 2007;426:23–28. - PubMed
-
- Axelsson A, Ringdahl A. Tinnitus—a study of its prevalence and characteristics. Br J Audiol. 1989;23:53–62. - PubMed
-
- Bartels H, Staal MJ, Albers FW. Tinnitus and neural plasticity of the brain. Otol Neurotol. 2007;28:178–184. - PubMed
-
- Baumer T, Lange R, Liepert J, Weiller C, Siebner HR, Rothwell JC, Munchau A. Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. Neuroimage. 2003;20:550–560. - PubMed
-
- Bilak M, Kim J, Potashner SJ, Bohne BA, Morest DK. New growth of axons in the cochlear nucleus of adult chinchillas after acoustic trauma. Exp Neurol. 1997;147:256–268. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

