Microcalorimetric method to assess phagocytosis: macrophage-nanoparticle interactions
- PMID: 21057907
- PMCID: PMC3032094
- DOI: 10.1208/s12248-010-9240-y
Microcalorimetric method to assess phagocytosis: macrophage-nanoparticle interactions
Abstract
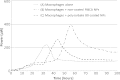
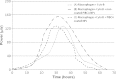
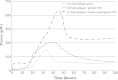
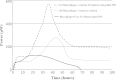
This study evaluated the use of isothermal microcalorimetry (ITMC) to detect macrophage-nanoparticle interactions. Four different nanoparticle (NP) formulations were prepared: uncoated poly(isobutyl cyanoacrylate) (PIBCA), polysorbate-80-coated PIBCA, gelatin, and mannosylated gelatin NPs. Changes in NP formulations were aimed to either enhance or decrease macrophage-NP interactions via phagocytosis. Alveolar macrophages were cultured on glass slabs and inserted in the ITMC instrument. Thermal activities of the macrophages alone and after titration of 100 μL of NP suspensions were compared. The relative interactive coefficients of macrophage-NP interactions were calculated using the heat exchange observed after NP titration. Control experiments were performed using cytochalasin B (Cyto B), a known phagocytosis inhibitor. The results of NP titration showed that the total thermal activity produced by macrophages changed according to the NP formulation. Mannosylated gelatin NPs were associated with the highest heat exchange, 75.4 ± 7.5 J, and thus the highest relative interactive coefficient, 9,269 ± 630 M-1. Polysorbate-80-coated NPs were associated with the lowest heat exchange, 15.2 ± 3.4 J, and the lowest interactive coefficient, 890 ± 120 M-1. Cyto B inhibited macrophage response to NPs, indicating a connection between the thermal activity recorded and NP phagocytosis. These results are in agreement with flow cytometry results. ITMC is a valuable tool to monitor the biological responses to nano-sized dosage forms such as NPs. Since the thermal activity of macrophage-NP interactions differed according to the type of NPs used, ITMC may provide a method to better understand phagocytosis and further the development of colloidal dosage forms.
Figures
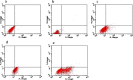
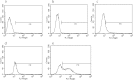
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

