Characterization of cytochrome P450 monooxygenase CYP154H1 from the thermophilic soil bacterium Thermobifida fusca
- PMID: 21057946
- PMCID: PMC3036808
- DOI: 10.1007/s00253-010-2965-9
Characterization of cytochrome P450 monooxygenase CYP154H1 from the thermophilic soil bacterium Thermobifida fusca
Abstract
Cytochrome P450 monooxygenases are valuable biocatalysts due to their ability to hydroxylate unactivated carbon atoms using molecular oxygen. We have cloned the gene for a new cytochrome P450 monooxygenase, named CYP154H1, from the moderately thermophilic soil bacterium Thermobifida fusca. The enzyme was overexpressed in Escherichia coli at up to 14% of total soluble protein and purified to homogeneity in three steps. CYP154H1 activity was reconstituted using putidaredoxin reductase and putidaredoxin from Pseudomonas putida DSM 50198 as surrogate electron transfer partners. In biocatalytic reactions with different aliphatic and aromatic substrates of varying size, the enzyme converted small aromatic and arylaliphatic compounds like ethylbenzene, styrene, and indole. Furthermore, CYP154H1 also accepted different arylaliphatic sulfides as substrates chemoselectively forming the corresponding sulfoxides and sulfones. The enzyme is moderately thermostable with an apparent melting temperature of 67°C and exhibited still 90% of initial activity after incubation at 50°C.
Figures



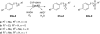
References
-
- Bell SG, Sowden RJ, Wong L-L. Engineering the haem monooxygenase cytochrome P450cam for monoterpene oxidation. Chem Comm. 2001;7:635–636. doi: 10.1039/b100290m. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

