The chemical basis of pharmacology
- PMID: 21058655
- PMCID: PMC2994275
- DOI: 10.1021/bi101540g
The chemical basis of pharmacology
Abstract
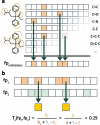
Molecular biology now dominates pharmacology so thoroughly that it is difficult to recall that only a generation ago the field was very different. To understand drug action today, we characterize the targets through which they act and new drug leads are discovered on the basis of target structure and function. Until the mid-1980s the information often flowed in reverse: investigators began with organic molecules and sought targets, relating receptors not by sequence or structure but by their ligands. Recently, investigators have returned to this chemical view of biology, bringing to it systematic and quantitative methods of relating targets by their ligands. This has allowed the discovery of new targets for established drugs, suggested the bases for their side effects, and predicted the molecular targets underlying phenotypic screens. The bases for these new methods, some of their successes and liabilities, and new opportunities for their use are described.
Figures
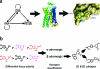
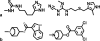
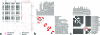
References
-
- Ahlquist R. P. (1948) A study of the adrenotropic receptors. Am. J. Physiol. 153, 586–600. - PubMed
-
- Lands A. M.; Arnold A.; McAuliff J. P.; Luduena F. P.; Brown T. G. Jr. (1967) Differentiation of receptor systems activated by sympathomimetic amines. Nature 214, 597–598. - PubMed
-
- Black J. W.; Duncan W. A.; Durant C. J.; Ganellin C. R.; Parsons E. M. (1972) Definition and antagonism of histamine H 2 -receptors. Nature 236, 385–390. - PubMed
-
- Richardson B. P.; Engel G.; Donatsch P.; Stadler P. A. (1985) Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature 316, 126–131. - PubMed
-
- Toll L.; Berzetei-Gurske I. P.; Polgar W. E.; Brandt S. R.; Adapa I. D.; Rodriguez L.; Schwartz R. W.; Haggart D.; O’Brien A.; White A.; Kennedy J. M.; Craymer K.; Farrington L.; Auh J. S. (1998) Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res. Monogr. 178, 440–466. - PubMed

