Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants
- PMID: 21058908
- PMCID: PMC3106242
- DOI: 10.1086/657309
Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants
Abstract
Background: Infants aged <12 months are at high risk of hospitalization for influenza. Influenza vaccine is recommended for pregnant women and for most children; however, no vaccine is approved for infants aged <6 months. Effective approaches are needed to protect this vulnerable population. Vaccination of women during pregnancy may protect the infant through transfer of antibodies from the mother. Few studies have examined the effectiveness of this strategy, and those studies produced mixed results.
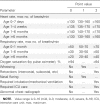
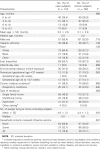
Methods: In a matched case-control study, case patients were infants aged <12 months admitted to a large urban hospital in the northeastern United States because of laboratory-confirmed influenza from 2000 to 2009. For each case, we enrolled 1 or 2 control subjects who were infants who tested negative for influenza and matched cases by date of birth and date of hospitalization (within 4 weeks). Vaccine effectiveness was calculated on the basis of matched odds ratios and was adjusted for confounding.
Results: The mothers of 2 (2.2%) of 91 case subjects and 31 (19.9%) of 156 control subjects aged <6 months, and 1 (4.6%) of 22 case subjects and 2 (5.6%) of 36 control subjects aged ≥6 months, had received influenza vaccine during pregnancy. The effectiveness of influenza vaccine given to mothers during pregnancy in preventing hospitalization among their infants, adjusted for potential confounders, was 91.5% (95% confidence interval [CI], 61.7%-98.1%; P = .001) for infants aged <6 months. The unadjusted effectiveness was 90.7% (95% CI, 59.9%-97.8%; P = .001).
Conclusions: Influenza vaccine given to pregnant women is 91.5% effective in preventing hospitalization of their infants for influenza in the first 6 months of life.
Figures
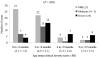


References
-
- Centers for Disease Control and Prevention. Public health and aging: influenza vaccination coverage among adults aged⩾50 years and pneumococcal vaccination coverage among adults aged ⩾65 years—United States, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:987–992. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. - PubMed
-
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–231. - PubMed
-
- Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58((RR-8)):1–52. - PubMed
-
- Gruber WC, Darden PM, Still JG, Lohr J, Reed G, Wright PF. Evaluation of bivalent live attenuated influenza A vaccines in children 2 months to 3 years of age: safety, immunogenicity and dose-response. Vaccine. 1997;15:1379–1384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

